|
 |
 |
 |
 |
 |
Novel structure of a human membrane enzyme sheds light on molecular mechanisms of rare ageing disorders and metabolic syndromes
Read about the structure of ZMPSTE24 in Science: Quigley et al., 2013 [Open Access: follow the link to the article in the new page].
Laminopathies are a group of rare genetic disorders caused by mutations in genes encoding proteins involved in the building and maintenance of the nuclei of cells. Severe laminopathies such as the premature ageing syndrome pregeria leads to death in the teens from heart disease and other symtoms normally found in people in their 80s. The structure of the nucleus is maintained by a class of proteins called lamins. When one of these proteins, prelamin A is not properly processed by an enzyme ZMPSTE24, laminopathies occur.
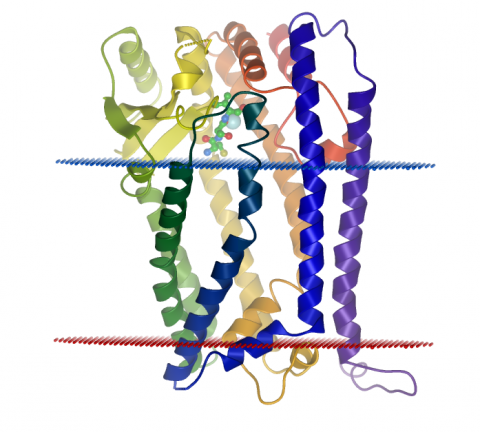
A group led by Dr. Liz Carpenter at the Structural Genomics Consortium have now mapped all the atoms that build ZMPSTE24 using a technique called X-ray crystallography and found a completely novel fold – the way in which the atoms in the enzyme are sequentially arranged.
Whilst the technique is very well established for most proteins, X-ray crystallography of novel human proteins embedded in cell membranes remains extremely challenging. In a very productive 2012 there were only 13 of such structures being generated in the World. Out of those, ZMPSTE24 – solved in Oxford, UK - is the only membrane-bound enzyme and the only novel human membrane protein structure solved in the whole of Europe.
Andrew Quigley, one of the primary authors of the study said: “My colleagues and I are extremely satisfied to be able to unveil and contemplate this fascinating structure for the first time and share our findings without any delay, to accelerate research in this field.”
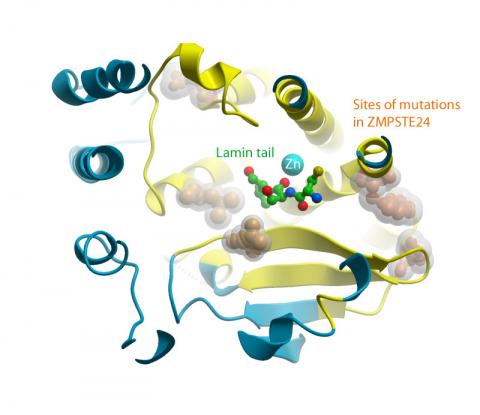
Laminopathy causing mutations in ZMPSTE24, shown in gold, surround the substrate binding site at the top of the cavity (click to enlarge)
As a result, scientists were able to see ZMPSTE24 assembled within the membrane of the nucleus. At its core a cavernous water-filled chamber spanning most of the nuclear membrane can be found. It is in this chamber that the tail end of prelamin A is processed by ZMPSTE24, which clips off a membrane binding tail from prelamin A. The crystal structure has also allowed scientists to see how changes (mutations) in the ZMPSTE24 enzyme could prevent the prelamin A tail from being removed and thus leave the protein stuck in the membrane – causing several diseases.
Liz Carpenter, the principal investigator who coordinated the project said: “The mapping of mutations in ZMPSTE24 that lead to laminopathies has implications not only for rare diseases such as premature ageing progerias, but also for normal ageing and metabolic syndromes.”
This work is part of the remit of the Structural Genomics Consortium (SGC), an Anglo-Canadian non-profit public-private partnership which aims to support the discovery of new medicines through open access research. As part of the SGC’s ethos all the information related to this research has been put in public domain immediately, allowing scientists across the World to freely work on it, hopefully advancing our understanding of several laminopathies.
The findings from this study has been published in the latest issue of the journal Science (29 March 2013) and is further discussed in a 'Perspective' article in the same issue by Susan Michaelis and Christine Hrycyna.
- Read the article in Science [Open Access: follow the link to the article in the new page]
- Read the 'Perspective' commentary about this work in Science
- Read more about the structures: 4AW6, 2YPT
- Watch the video: Structure of Human ZMPSTE24