|
 |
 |
 |
 |
 |
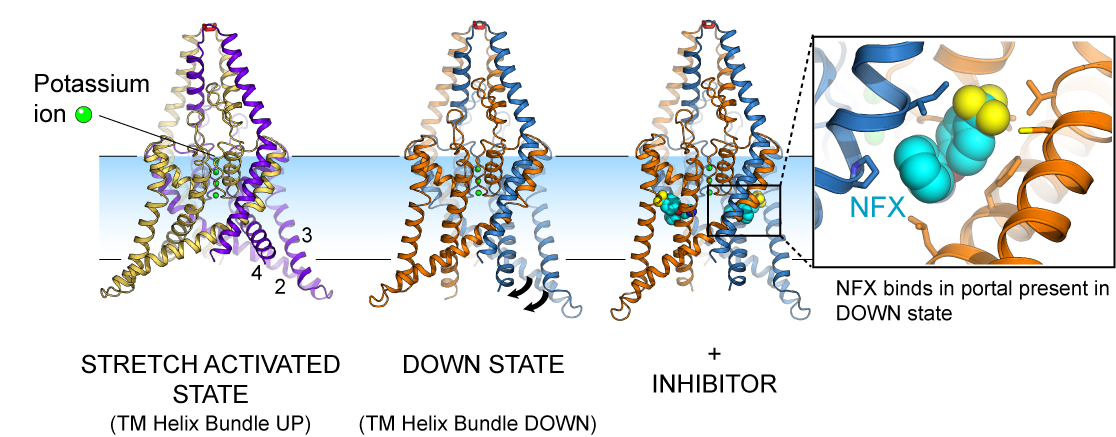
Structure of the TREK-2 ion channel shows how nerves sense touch and respond to drugs
The question of how nerves sense touch, pressure and pain has been a long standing question in physiology. Also the question of how drugs can affect the nerve’s ability to feel pain is critical for design of drugs that will influence our perception of pain. In order to understand how we sense pressure and pain, Assoc. Prof. Liz Carpenter’s group at the SGC, in collaboration with Assoc. Prof. Stephen Tucker in Physics and Prof. Mark Sansom in Biochemistry have looked at a family of human ion channels. These are proteins in nerve membranes that are sensitive to stimuli such as the stretching of a membrane, thus allowing nerves to detect stretch.
In fact these polymodal channels, called TREK channels, respond to a host of different stimuli, including heat, stretch, pH and changes in lipids in cell membranes. In vitro experiments have shown that TREK channels are sensitive to a range of existing drugs and anaesthetics, which can be modified to create potential new drugs that targeting TREK channels in humans.
At the SGC Liz Carpenter’s group used X-ray crystallography, a diffraction technique, to solve the structure of the channel TREK-2 in two conformations. TREK-2 sits in the lipid bilayers that form the membrane around nerve cells. They allow potassium ions to flow down a charge and concentration gradient when the channels are open. The channels form a selectivity filter which binds three or four potassium ions and flow of the ions through the selectivity filter is controlled by changes in remote parts of the structure, in particular a series of three helices that move up and down in the membrane. When the membrane is not stretched the helices project further into the cell, whereas stretching of the membrane would bring the helices up onto the membrane, allowing the channel to open, causing the nerve to fire and the sensation of touch to be felt. The SGC and other group[1] have observed these movements. When the channel is in the “down” state, in an unstretched membrane conformation, then small molecules, such as norfluoxetine (the active ingredient of Prozac) can bind in a portal in the side of the protein. In the stretched state the portal disappears, so when the drug is bound, the protein can no longer go into the stretched, more active state.
We have active collaborations with colleagues in Oxford to use electrophysiology and mutagenesis to study the effects predicted by the changes in channel structure (Stephen Tucker’s group, Dept. of Physics), as well as computational methods to study structural changes of TREK-2 and other K2P ion channels in silico (Mark Sansom’s group, Dept. of Biochemistry).
Together these methods have allowed us to begin to understand, at a molecular level, how channels that respond to a range of different stimuli, including stretch and small molecule drugs, lead to changes in nerve activity. This tells us how nerves can respond to pressure and pain, and how drugs could be designed to modify this process.
More information:
- Human two-pore domain potassium ion channel TREK2 (K2P10.1)
- Science (Open Access): K2P channel gating mechanisms revealed by structures of TREK-2 and a complex with Prozac
- How an ion channel converts mechanical force into an electrical signal
- [1] Physical mechanism for gating and mechanosensitivity of the human TRAAK K+channel Brohawn, S.G., Campbell, E.B., Mackinnon, R. (2014)- Nature 516, 126-130.