The probe and control may be requested here.
| Probe | Negative control | |
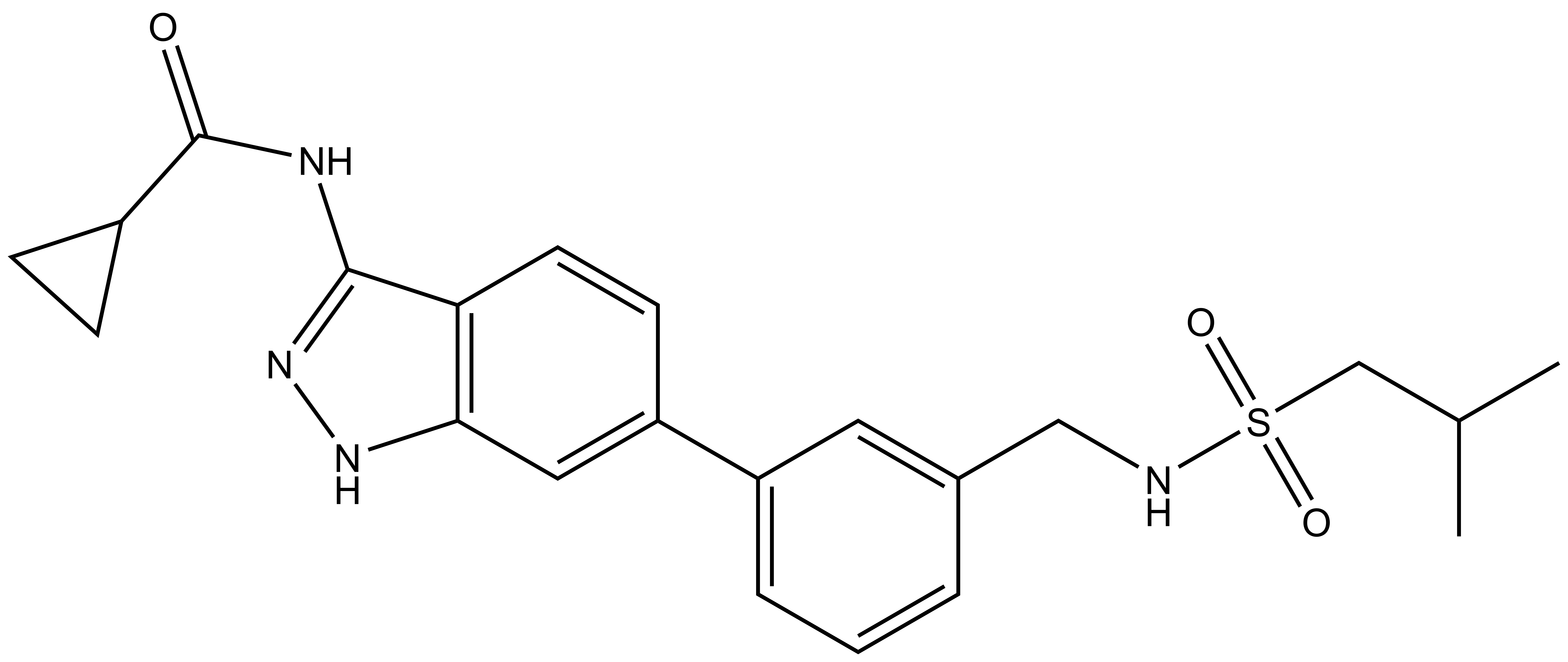 | 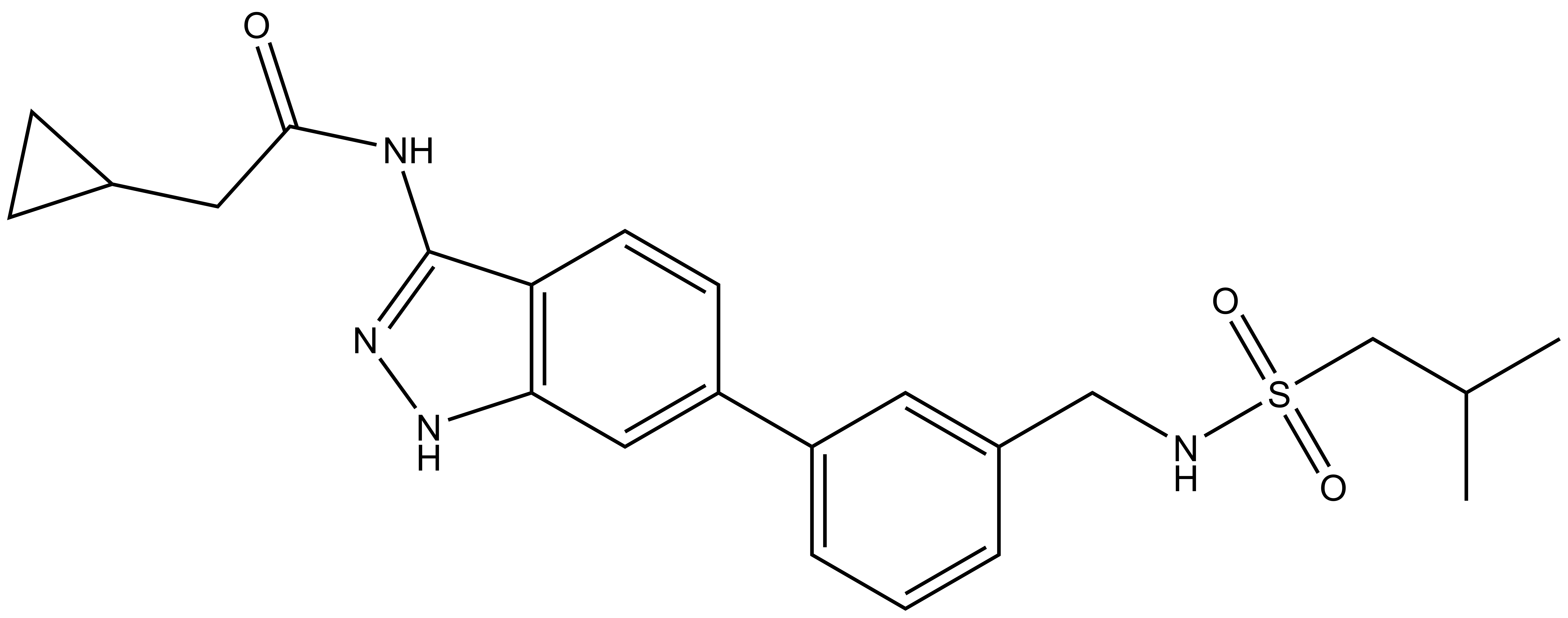 | |
SGC-CDKL2/AAK1/BMP2K-1 | CDKL2/AAK1/BMP2K -1N |
From a library of acylaminoindazoles, we identified a potent and cell-active chemical probe (SGC-CDKL2/AAK1/BMP2K-1) that inhibits cyclin-dependent kinase-like 2 (CDKL2). Comprehensive evaluation of kinome-wide selectivity confirmed that this chemical probe demonstrates good selectivity. A structurally similar analog(SGC-CDKL2/AAK1/BMP2K-1N) was characterized as a negative control that does not bind to CDKL2, AAK1, or BMP2K in corresponding cellular target engagement assays. SGC-CDKL2/AAK1/BMP2K-1 is devoid of AAK1 and BMP2K inhibition when used at an appropriate concentration (≤1 µM in cells). At biologically relevant concentrations (≤1 µM), our chemical probe inhibited EB2 phosphorylation in rat primary neurons. A chemical probe that inhibits AAK1 and BMP2K, SGC-AAK1-1, represents a compound that can be profiled in parallel to discern whether an observed phenotype is driven by CDKL2 inhibition. When used at ≤1 µM in cell-based assays, our chemical probe set can help the community further characterize the underexplored roles of CDKL2.
Biological activity summary:
| Physical and chemical properties for SGC-CDKL2/AAK1/BMP2K-1 | |
| Molecular weight | 426.54 |
| Molecular formula | C22H26N4O3S |
| IUPAC name | N-(6-(3-(((2-methylpropyl)sulfonamido)methyl)phenyl)-1H-indazol-3-yl)cyclopropanecarboxamide |
| ClogP | 3.24 |
| PSA | 112.33 |
| No. of chiral centers | 0 |
| No. of rotatable bonds | 9 |
| No. of hydrogen bond acceptors | 5 |
| No. of hydrogen bond donors | 3 |
| Storage | Stable as a solid at room temperature. DMSO stock solutions (up to 10 mM) are stable at -20oC |
| Dissolution | Soluble in DMSO up to 10 mM |
| Physical and chemical properties for SGC-CDKL2/AAK1/BMP2K-1N | |
| Molecular weight | 440.56 |
| Molecular formula | C23H28N4O3S |
| IUPAC name | 2-cyclopropyl-N-(6-(3-(((2-methylpropyl)sulfonamido)methyl)phenyl)-1H-indazol-3-yl)acetamide |
| ClogP | 3.63 |
| PSA | 112.33 |
| No. of chiral centers | 0 |
| No. of rotatable bonds | 10 |
| No. of hydrogen bond acceptors | 5 |
| No. of hydrogen bond donors | 3 |
| Storage | Stable as a solid at room temperature. DMSO stock solutions (up to 10 mM) are stable at -20oC |
| Dissolution | Soluble in DMSO up to 10 mM |
SGC-CDKL2/AAK1/BMP2K-1 was profiled in the DiscoverX MAX assay against 403 wild-type kinases at 1 μM. Only 2 kinases showed PoC <10 giving an S10(1 μM) = 0.002. When the PoC <40 fraction was examined, 10 kinases were included (S35(1 μM) = 0.022). Potential off-targets within the S40(1 μM) fraction were tested via biochemical enzymatic and/or NanoBRET target engagement assays. Data corresponding with off-target kinase activity is shown in the table below.
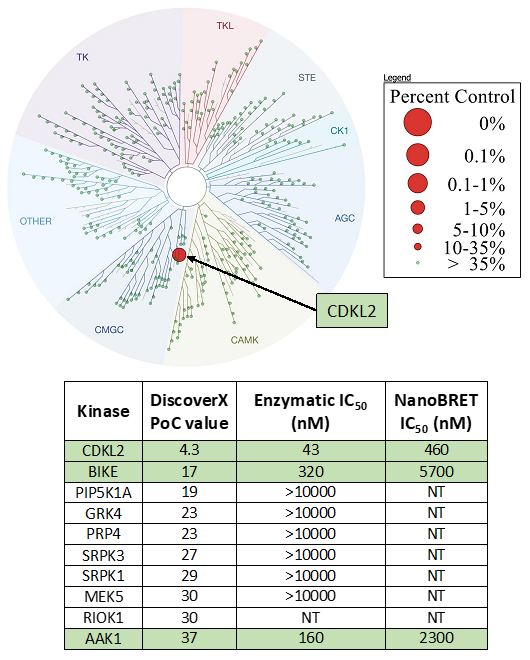
Figure 2: SGC-CDKL2/AAK1/BMP2K-1 was profiled in the DiscoverX MAX assay against 403 wild-type kinases at 1 μM and off-target kinases inhibited PoC <40 were tested in an orthogonal assay. Rows colored green correspond with CDKL2, AAK1, and BMP2K. No other kinases demonstrate enzymatic IC50 values within 30-fold of the CDKL2 enzymatic assay IC50 value.
A NanoBRET assay was utilized to assess the binding affinity of SGC-CDKL2/AAK1/BMP2K-1 to CDKL2, AAK1, and BMP2K. The negative control shows no binding affinity for CDKL2, AAK1, or BMP2K.
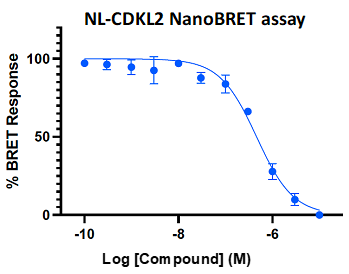
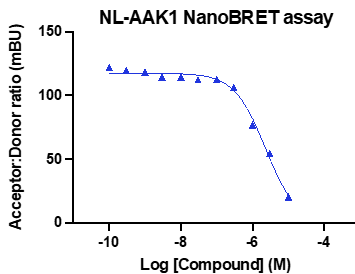
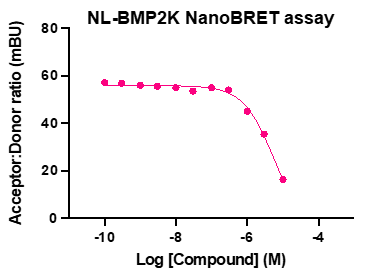
Figure 3: SGC-CDKL2/AAK1/BMP2K-1 was profiled in the CDKL2, AAK1, and BMP2K NanoBRET assays.
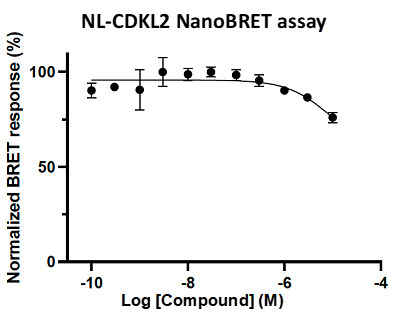
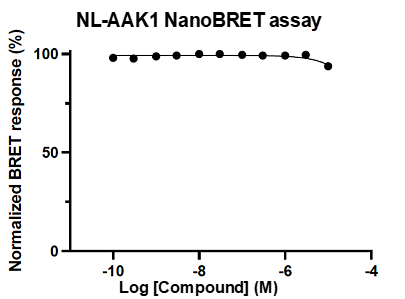
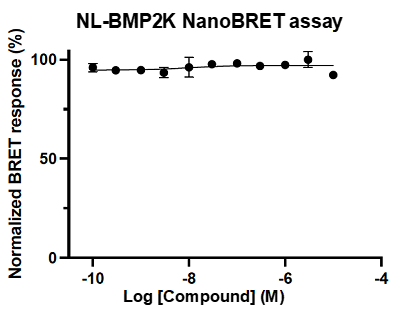
Figure 4: SGC-CDKL2/AAK1/BMP2K-1N was profiled in the CDKL2, AAK1, and BMP2K NanoBRET assays.
Bashore, F. M.; Min, S. M.; Chen, X.; Howell, S.; Rinderle, C. H.; Morel, G.; Silvaroli, J. A.; Wells, C. I.; Bunnell, B. A.; Drewry, D. H.; Pabla, N. S.; Ultanir, S. K.; Bullock, A. N.; Axtman, A D. Discovery and Characterization of a Chemical Probe for Cyclin-Dependent Kinase-Like 2. ACS Med Chem Lett , doi: 10.1021/acsmedchemlett.4c00219.
Bashore, F. M.; Min, S. M.; Chen, X.; Howell, S.; Rinderle, C. H.; Morel, G.; Silvaroli, J. A.; Wells, C. I.; Bunnell, B. A.; Drewry, D. H.; Pabla, N. S.; Ultanir, S. K.; Bullock, A. N.; Axtman, A D. Discovery and Characterization of a Chemical Probe for Cyclin-Dependent Kinase-Like 2. BioRxiv, doi: 10.1101/2024.05.12.593776.