
Dr. Dalia Barsyte-Lovejoy, PhD is an Assistant Professor at the Department of Pharmacology and Toxicology, UofT, and Principal Investigator at the SGC-Toronto, working to understand fundamental regulatory mechanisms of epigenetic proteins and their pharmacological modulation in cancer. The group’s research focuses on disease mechanisms, therapeutic targets, and chemical probe discovery, resulting in over 30 extensively characterized compounds that have helped shape the emerging field of epigenetics and enabled over 50 collaborative projects that are uncovering new epigenetic mechanisms in cancer and its treatment.
We are interested in understanding the mechanism of epigenetic regulators and posttranslational modifications that control cancer cell growth, differentiation, and therapy response. Protein lysine and arginine methyltransferases regulate transcription, genome stability, splicing, RNA metabolism, and other cell processes dictated by which substrates these enzymes methylate. Lysine methyltransferases such as EZH2 and NSD2 primarily methylate histones to establish repressive and active chromatin. In contrast, arginine methyltransferases have a broad scope of substrates ranging from histones to signaling molecules, enzymes, and structural proteins. Epigenetic chromatin regulation, transcriptome, and cellular signaling are fine-tuned by ubiquitin modification. Our work seeks to understand how these posttranslational modifications are misregulated in cancer and identify new therapeutic targets.
Through multidisciplinary research that includes cell and chemical biology, protein structural biology, and many collaborative studies with colleagues across industry and academia, the SGC chemical probes project has generated several probes for methyltransferases, ubiquitin ligases, and deubiquitylases. We are currently using these chemical probes to explore the cellular pathways in poor prognosis acute myeloid leukemia, pancreatic, lung and breast cancer.
Chemical probes as tools for cancer target discovery
|
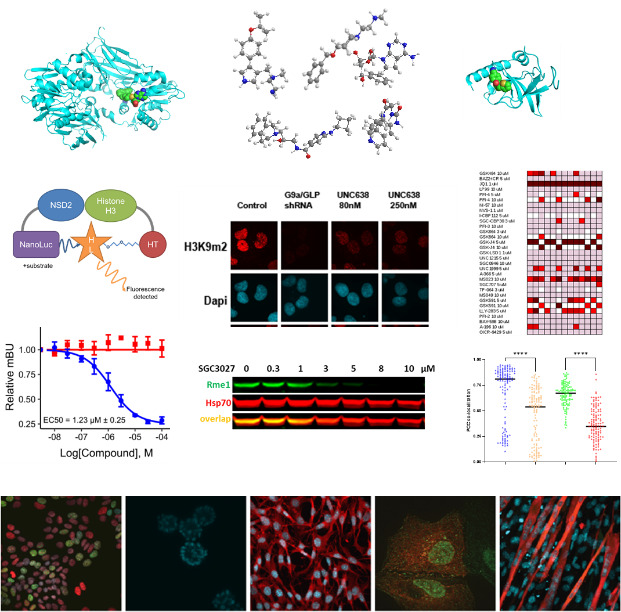
To study epigenetic modifier proteins, we need genetic and pharmacological tools. Chemical probe compounds that potently and selectively inhibit or degrade the target proteins in cells provide tools for modulating activating/repressing histone marks and other cellular signaling pathways. By discovering and using chemical probes, we expand our understanding of the protein function and its therapeutic utility to establish a biological rationale in cancer therapy.
|
Link to Open Lab notebooks that features science community posts on our various projects https://openlabnotebooks.org/
Hanley RP, Nie DY, Tabor JR, Li F, Sobh A, Xu C, Barker NK, Dilworth D, Hajian T, Gibson E, Szewczyk MM, Brown PJ, Barsyte-Lovejoy D, Herring LE, Wang GG, Licht JD, Vedadi M, Arrowsmith CH, James LI
J Am Chem Soc. 2023-3-28 . .doi: 10.1021/jacs.3c01421
PMID: 36976643Li ASM, Kimani S, Wilson B, Noureldin M, González-Álvarez H, Mamai A, Hoffer L, Guilinger JP, Zhang Y, von Rechenberg M, Disch JS, Mulhern CJ, Slakman BL, Cuozzo JW, Dong A, Poda G, Mohammed M, Saraon P, Mittal M, Modh P, Rathod V, Patel B, Ackloo S, Santhakumar V, Szewczyk MM, Barsyte-Lovejoy D, Arrowsmith CH, Marcellus R, Guié MA, Keefe AD, Brown PJ, Halabelian L, Al-Awar R, Vedadi M
J Med Chem. 2023-3-22 . .doi: 10.1021/acs.jmedchem.2c02132
PMID: 36948210Dahlin JL, Hua BK, Zucconi BE, Nelson SD, Singh S, Carpenter AE, Shrimp JH, Lima-Fernandes E, Wawer MJ, Chung LPW, Agrawal A, O'Reilly M, Barsyte-Lovejoy D, Szewczyk M, Li F, Lak P, Cuellar M, Cole PA, Meier JL, Thomas T, Baell JB, Brown PJ, Walters MA, Clemons PA, Schreiber SL, Wagner BK
Nat Commun. 2023-3-13 . 14(1):1364 .doi: 10.1038/s41467-023-36829-x
PMID: 36914634Göricke F, Vu V, Smith L, Scheib U, Böhm R, Akkilic N, Wohlfahrt G, Weiske J, Bömer U, Brzezinka K, Lindner N, Lienau P, Gradl S, Beck H, Brown PJ, Santhakumar V, Vedadi M, Barsyte-Lovejoy D, Arrowsmith CH, Schmees N, Petersen K
J Med Chem. 2023-2-20 . .doi: 10.1021/acs.jmedchem.2c01933
PMID: 36802665Barsyte-Lovejoy D
J Vis Exp. 2023-1-20 . .doi: 10.3791/64821
PMID: 37602846Hogg DW, Reid AL, Dodsworth TL, Chen Y, Reid RM, Xu M, Husic M, Biga PR, Slee A, Buck LT, Barsyte-Lovejoy D, Locke M, Lovejoy DA
Front Physiol. 2022-12-17 . 13:1031264 .doi: 10.3389/fphys.2022.1031264
PMID: 36523555Barghout SH, Mann MK, Aman A, Yu Y, Alteen MG, Schimmer AD, Schapira M, Arrowsmith CH, Barsyte-Lovejoy D
ACS Chem Biol. 2022-9-9 . .doi: 10.1021/acschembio.2c00451
PMID: 36084291Trush VV, Feller C, Li ASM, Allali-Hassani A, Szewczyk MM, Chau I, Eram MS, Jiang B, Luu R, Zhang F, Barsyte-Lovejoy D, Aebersold R, Arrowsmith CH, Vedadi M
Biochim Biophys Acta Gene Regul Mech. 2022-7-27 . 194845 .doi: 10.1016/j.bbagrm.2022.194845
PMID: 35907431Barghout SH, Machado RAC, Barsyte-Lovejoy D
Biochim Biophys Acta Gene Regul Mech. 2022-6-23 . 194840 .doi: 10.1016/j.bbagrm.2022.194840
PMID: 35753676Wu Q, Nie DY, Ba-Alawi W, Ji Y, Zhang Z, Cruickshank J, Haight J, Ciamponi FE, Chen J, Duan S, Shen Y, Liu J, Marhon SA, Mehdipour P, Szewczyk MM, Dogan-Artun N, Chen W, Zhang LX, Deblois G, Prinos P, Massirer KB, Barsyte-Lovejoy D, Jin J, De Carvalho DD, Haibe-Kains B, Wang X, Cescon DW, Lupien M, Arrowsmith CH
Nat Chem Biol. 2022-5-16 . .doi: 10.1038/s41589-022-01024-4
PMID: 35578032