
Dr. Dalia Barsyte-Lovejoy, PhD is an Assistant Professor at the Department of Pharmacology and Toxicology, UofT, and Principal Investigator at the SGC-Toronto, working to understand fundamental regulatory mechanisms of epigenetic proteins and their pharmacological modulation in cancer. The group’s research focuses on disease mechanisms, therapeutic targets, and chemical probe discovery, resulting in over 30 extensively characterized compounds that have helped shape the emerging field of epigenetics and enabled over 50 collaborative projects that are uncovering new epigenetic mechanisms in cancer and its treatment.
We are interested in understanding the mechanism of epigenetic regulators and posttranslational modifications that control cancer cell growth, differentiation, and therapy response. Protein lysine and arginine methyltransferases regulate transcription, genome stability, splicing, RNA metabolism, and other cell processes dictated by which substrates these enzymes methylate. Lysine methyltransferases such as EZH2 and NSD2 primarily methylate histones to establish repressive and active chromatin. In contrast, arginine methyltransferases have a broad scope of substrates ranging from histones to signaling molecules, enzymes, and structural proteins. Epigenetic chromatin regulation, transcriptome, and cellular signaling are fine-tuned by ubiquitin modification. Our work seeks to understand how these posttranslational modifications are misregulated in cancer and identify new therapeutic targets.
Through multidisciplinary research that includes cell and chemical biology, protein structural biology, and many collaborative studies with colleagues across industry and academia, the SGC chemical probes project has generated several probes for methyltransferases, ubiquitin ligases, and deubiquitylases. We are currently using these chemical probes to explore the cellular pathways in poor prognosis acute myeloid leukemia, pancreatic, lung and breast cancer.
Chemical probes as tools for cancer target discovery
|
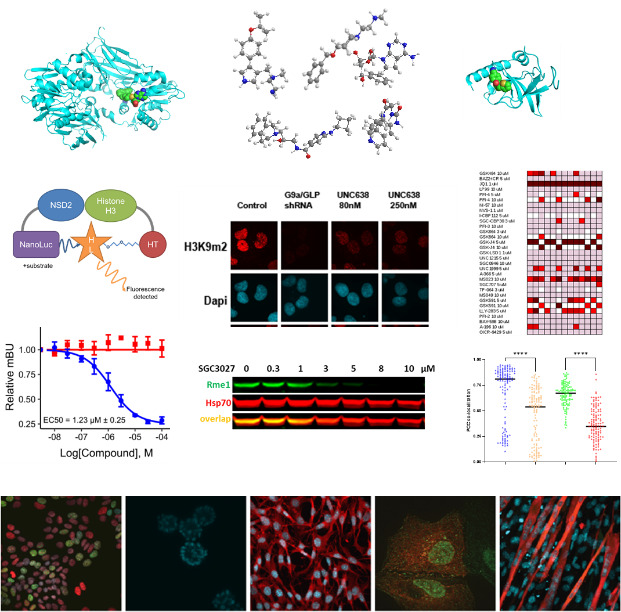
To study epigenetic modifier proteins, we need genetic and pharmacological tools. Chemical probe compounds that potently and selectively inhibit or degrade the target proteins in cells provide tools for modulating activating/repressing histone marks and other cellular signaling pathways. By discovering and using chemical probes, we expand our understanding of the protein function and its therapeutic utility to establish a biological rationale in cancer therapy.
|
Link to Open Lab notebooks that features science community posts on our various projects https://openlabnotebooks.org/
Shen Y, Li F, Szewczyk MM, Halabelian L, Chau I, Eram MS, Dela Seña C, Park KS, Meng F, Chen H, Zeng H, McLeod D, Zepeda-Velázquez CA, Campbell RM, Mader MM, Watson BM, Schapira M, Arrowsmith CH, Al-Awar R, Barsyte-Lovejoy D, Kaniskan HÜ, Brown PJ, Vedadi M, Jin J
J Med Chem. 2021-2-16 . .doi: 10.1021/acs.jmedchem.0c02160
PMID: 33591753Sachamitr P, Ho JC, Ciamponi FE, Ba-Alawi W, Coutinho FJ, Guilhamon P, Kushida MM, Cavalli FMG, Lee L, Rastegar N, Vu V, Sánchez-Osuna M, Coulombe-Huntington J, Kanshin E, Whetstone H, Durand M, Thibault P, Hart K, Mangos M, Veyhl J, Chen W, Tran N, Duong BC, Aman AM, Che X, Lan X, Whitley O, Zaslaver O, Barsyte-Lovejoy D, Richards LM, Restall I, Caudy A, Röst HL, Bonday ZQ, Bernstein M, Das S, Cusimano MD, Spears J, Bader GD, Pugh TJ, Tyers M, Lupien M, Haibe-Kains B, Artee Luchman H, Weiss S, Massirer KB, Prinos P, Arrowsmith CH, Dirks PB
Nat Commun. 2021-2-12 . 12(1):979 .doi: 10.1038/s41467-021-21204-5
PMID: 33579912Ferreira de Freitas R, Liu Y, Szewczyk MM, Mehta N, Li F, McLeod D, Zepeda-Velázquez C, Dilworth D, Hanley RP, Gibson E, Brown PJ, Al-Awar R, James LI, Arrowsmith CH, Barsyte-Lovejoy D, Min J, Vedadi M, Schapira M, Allali-Hassani A
J Med Chem. 2021-2-1 . .doi: 10.1021/acs.jmedchem.0c01768
PMID: 33522809Szewczyk MM, Ishikawa Y, Organ S, Sakai N, Li F, Halabelian L, Ackloo S, Couzens AL, Eram M, Dilworth D, Fukushi H, Harding R, Dela Seña CC, Sugo T, Hayashi K, McLeod D, Zepeda C, Aman A, Sánchez-Osuna M, Bonneil E, Takagi S, Al-Awar R, Tyers M, Richard S, Takizawa M, Gingras AC, Arrowsmith CH, Vedadi M, Brown PJ, Nara H, Barsyte-Lovejoy D
Nat Commun. 2020-5-14 . 11(1):2396 .doi: 10.1038/s41467-020-16271-z
PMID: 32409666Shen Y, Li F, Szewczyk MM, Halabelian L, Park KS, Chau I, Dong A, Zeng H, Chen H, Meng F, Barsyte-Lovejoy D, Arrowsmith CH, Brown P, Liu J, Vedadi M, Jin J
J. Med. Chem.. 2020-5-5 . .doi: 10.1021/acs.jmedchem.0c00406
PMID: 32367723Criqui M, Qamra A, Chu TW, Sharma M, Tsao J, Henry DA, Barsyte-Lovejoy D, Arrowsmith CH, Winegarden N, Lupien M, Harrington L
Elife. 2020-4-16 . 9: .doi: 10.7554/eLife.47333
PMID: 32297856Allali-Hassani A, Szewczyk MM, Ivanochko D, Organ SL, Bok J, Ho JSY, Gay FPH, Li F, Blazer L, Eram MS, Halabelian L, Dilworth D, Luciani GM, Lima-Fernandes E, Wu Q, Loppnau P, Palmer N, Talib SZA, Brown PJ, Schapira M, Kaldis P, O'Hagan RC, Guccione E, Barsyte-Lovejoy D, Arrowsmith CH, Sanders JM, Kattar SD, Bennett DJ, Nicholson B, Vedadi M
Nat Commun. 2019-12-17 . 10(1):5759 .doi: 10.1038/s41467-019-13652-x
PMID: 31848333Lovejoy DA, Hogg DW, Dodsworth TL, Jurado FR, Read CC, D'Aquila AL, Barsyte-Lovejoy D
Front Endocrinol (Lausanne). 2019-11-30 . 10:730 .doi: 10.3389/fendo.2019.00730
PMID: 31781029Barsyte-Lovejoy D
Stem Cell Investig. 2019-9-29 . 6:20 .doi: 10.21037/sci.2019.06.01
PMID: 31559307Taylor AP, Szewczyk MM, Kennedy S, Trush VV, Wu H, Zeng H, Dong A, de Freitas RF, Tatlock JH, Kumpf RA, Wythes M, Casimiro-Garcia A, Denny RA, Parikh MD, Li F, Barsyte-Lovejoy D, Schapira M, Vedadi M, Brown P, Arrowsmith CH, Owen DR
J. Med. Chem.. 2019-8-15 . .doi: 10.1021/acs.jmedchem.9b00112
PMID: 31415173