
Dr. Dalia Barsyte-Lovejoy, PhD is an Assistant Professor at the Department of Pharmacology and Toxicology, UofT, and Principal Investigator at the SGC-Toronto, working to understand fundamental regulatory mechanisms of epigenetic proteins and their pharmacological modulation in cancer. The group’s research focuses on disease mechanisms, therapeutic targets, and chemical probe discovery, resulting in over 30 extensively characterized compounds that have helped shape the emerging field of epigenetics and enabled over 50 collaborative projects that are uncovering new epigenetic mechanisms in cancer and its treatment.
We are interested in understanding the mechanism of epigenetic regulators and posttranslational modifications that control cancer cell growth, differentiation, and therapy response. Protein lysine and arginine methyltransferases regulate transcription, genome stability, splicing, RNA metabolism, and other cell processes dictated by which substrates these enzymes methylate. Lysine methyltransferases such as EZH2 and NSD2 primarily methylate histones to establish repressive and active chromatin. In contrast, arginine methyltransferases have a broad scope of substrates ranging from histones to signaling molecules, enzymes, and structural proteins. Epigenetic chromatin regulation, transcriptome, and cellular signaling are fine-tuned by ubiquitin modification. Our work seeks to understand how these posttranslational modifications are misregulated in cancer and identify new therapeutic targets.
Through multidisciplinary research that includes cell and chemical biology, protein structural biology, and many collaborative studies with colleagues across industry and academia, the SGC chemical probes project has generated several probes for methyltransferases, ubiquitin ligases, and deubiquitylases. We are currently using these chemical probes to explore the cellular pathways in poor prognosis acute myeloid leukemia, pancreatic, lung and breast cancer.
Chemical probes as tools for cancer target discovery
|
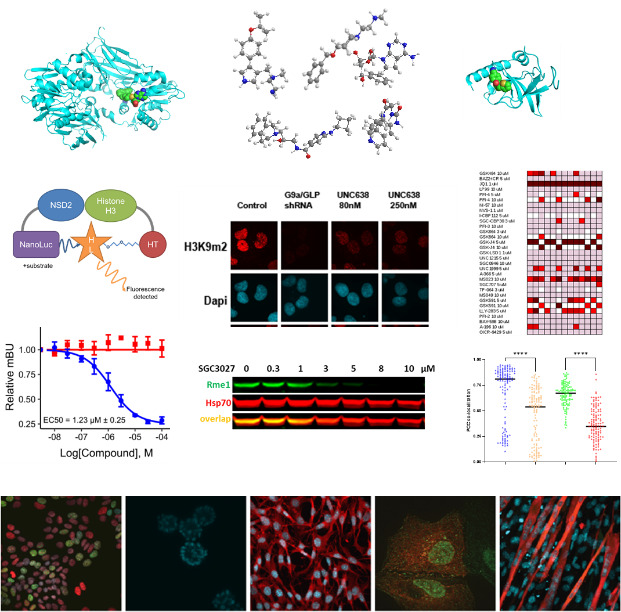
To study epigenetic modifier proteins, we need genetic and pharmacological tools. Chemical probe compounds that potently and selectively inhibit or degrade the target proteins in cells provide tools for modulating activating/repressing histone marks and other cellular signaling pathways. By discovering and using chemical probes, we expand our understanding of the protein function and its therapeutic utility to establish a biological rationale in cancer therapy.
|
Link to Open Lab notebooks that features science community posts on our various projects https://openlabnotebooks.org/
Bonday ZQ, Cortez GS, Grogan MJ, Antonysamy S, Weichert K, Bocchinfuso WP, Li F, Kennedy S, Li B, Mader MM, Arrowsmith CH, Brown PJ, Eram MS, Szewczyk MM, Barsyte-Lovejoy D, Vedadi M, Guccione E, Campbell RM
ACS Med Chem Lett. 2018-7-12 . 9(7):612-617 .doi: 10.1021/acsmedchemlett.8b00014
PMID: 30034588Casey AE, Sinha A, Singhania R, Livingstone J, Waterhouse P, Tharmapalan P, Cruickshank J, Shehata M, Drysdale E, Fang H, Kim H, Isserlin R, Bailey S, Medina T, Deblois G, Shiah YJ, Barsyte-Lovejoy D, Hofer S, Bader G, Lupien M, Arrowsmith C, Knapp S, De Carvalho D, Berman H, Boutros PC, Kislinger T, Khokha R
J. Cell Biol.. 2018-6-19 . .doi: 10.1083/jcb.201804042
PMID: 29921600Nakayama K, Szewczyk MM, Dela Sena C, Wu H, Dong A, Zeng H, Li F, de Freitas RF, Eram MS, Schapira M, Baba Y, Kunitomo M, Cary DR, Tawada M, Ohashi A, Imaeda Y, Saikatendu KS, Grimshaw CE, Vedadi M, Arrowsmith CH, Barsyte-Lovejoy D, Kiba A, Tomita D, Brown PJ
Oncotarget. 2018-4-6 . 9(26):18480-18493 .doi: 10.18632/oncotarget.24883
PMID: 29719619Kaniskan HÜ, Eram MS, Zhao K, Szewczyk MM, Yang X, Schmidt K, Luo X, Xiao S, Dai M, He F, Zang I, Lin Y, Li F, Dobrovetsky E, Smil D, Min SJ, Lin-Jones J, Schapira M, Atadja P, Li E, Barsyte-Lovejoy D, Arrowsmith CH, Brown PJ, Liu F, Yu Z, Vedadi M, Jin J
J. Med. Chem.. 2017-12-15 . .doi: 10.1021/acs.jmedchem.7b01674
PMID: 29244490Dahlin JL, Nelson KM, Strasser JM, Barsyte-Lovejoy D, Szewczyk MM, Organ S, Cuellar M, Singh G, Shrimp JH, Nguyen N, Meier JL, Arrowsmith CH, Brown PJ, Baell JB, Walters MA
Nat Commun. 2017-11-15 . 8(1):1527 .doi: 10.1038/s41467-017-01657-3
PMID: 29142305Vedadi M, Blazer L, Eram MS, Barsyte-Lovejoy D, Arrowsmith CH, Hajian T
Protein Sci.. 2017-2-4 . .doi: 10.1002/pro.3129
PMID: 28160335He Y, Selvaraju S, Curtin ML, Jakob CG, Zhu H, Comess KM, Shaw B, The J, Lima-Fernandes E, Szewczyk MM, Cheng D, Klinge KL, Li HQ, Pliushchev M, Algire MA, Maag D, Guo J, Dietrich J, Panchal SC, Petros AM, Sweis RF, Torrent M, Bigelow LJ, Senisterra G, Li F, Kennedy S, Wu Q, Osterling DJ, Lindley DJ, Gao W, Galasinski S, Barsyte-Lovejoy D, Vedadi M, Buchanan FG, Arrowsmith CH, Chiang GG, Sun C, Pappano WN
Nat. Chem. Biol.. 2017-1-30 . .doi: 10.1038/nchembio.2306
PMID: 28135237Bromberg KD, Mitchell TR, Upadhyay AK, Jakob CG, Jhala MA, Comess KM, Lasko LM, Li C, Tuzon CT, Dai Y, Li F, Eram MS, Nuber A, Soni NB, Manaves V, Algire MA, Sweis RF, Torrent M, Schotta G, Sun C, Michaelides MR, Shoemaker AR, Arrowsmith CH, Brown PJ, Santhakumar V, Martin A, Rice JC, Chiang GG, Vedadi M, Barsyte-Lovejoy D, Pappano WN
Nat. Chem. Biol.. 2017-1-23 . .doi: 10.1038/nchembio.2282
PMID: 28114273Torchia J, Golbourn B, Feng S, Ho KC, Sin-Chan P, Vasiljevic A, Norman JD, Guilhamon P, Garzia L, Agamez NR, Lu M, Chan TS, Picard D, de Antonellis P, Khuong-Quang DA, Planello AC, Zeller C, Barsyte-Lovejoy D, Lafay-Cousin L, Letourneau L, Bourgey M, Yu M, Gendoo DM, Dzamba M, Barszczyk M, Medina T, Riemenschneider AN, Morrissy AS, Ra YS, Ramaswamy V, Remke M, Dunham CP, Yip S, Ng HK, Lu JQ, Mehta V, Albrecht S, Pimentel J, Chan JA, Somers GR, Faria CC, Roque L, Fouladi M, Hoffman LM, Moore AS, Wang Y, Choi SA, Hansford JR, Catchpoole D, Birks DK, Foreman NK, Strother D, Klekner A, Bognár L, Garami M, Hauser P, Hortobágyi T, Wilson B, Hukin J, Carret AS, Van Meter TE, Hwang EI, Gajjar A, Chiou SH, Nakamura H, Toledano H, Fried I, Fults D, Wataya T, Fryer C, Eisenstat DD, Scheinemann K, Fleming AJ, Johnston DL, Michaud J, Zelcer S, Hammond R, Afzal S, Ramsay DA, Sirachainan N, Hongeng S, Larbcharoensub N, Grundy RG, Lulla RR, Fangusaro JR, Druker H, Bartels U, Grant R, Malkin D, McGlade CJ, Nicolaides T, Tihan T, Phillips J, Majewski J, Montpetit A, Bourque G, Bader GD, Reddy AT, Gillespie GY, Warmuth-Metz M, Rutkowski S, Tabori U, Lupien M, Brudno M, Schüller U, Pietsch T, Judkins AR, Hawkins CE, Bouffet E, Kim SK, Dirks PB, Taylor MD, Erdreich-Epstein A, Arrowsmith CH, De Carvalho DD, Rutka JT, Jabado N, Huang A
Cancer Cell. 2016-12-12 . 30(6):891-908 .doi: 10.1016/j.ccell.2016.11.003
PMID: 27960086Shen Y, Szewczyk MM, Eram MS, Smil D, Kaniskan HÜ, de Freitas RF, Senisterra G, Li F, Schapira M, Brown PJ, Arrowsmith CH, Barsyte-Lovejoy D, Liu J, Vedadi M, Jin J
J. Med. Chem.. 2016-9-1 . .doi: 10.1021/acs.jmedchem.6b01033
PMID: 27584694