 |
 |
 |
 |
 |
 |
Opening the gates for a new therapeutic opportunity in the fight against apicomplexan parasites
 Apicomplexan parasites are a diverse group of protozoan parasites, several of which cause important human and animal diseases, such as malaria, cryptosporidiosis and toxoplasmosis. Many of these diseases are endemic in developing countries, which are impacted by the lack of cost-efficient treatments. Cryptosporidiosis and toxoplasmosis can also be life-threatening to immunocompromised individuals, e.g. those undergoing organ transplantation or chemotherapy and HIV/AIDS patients, who cannot fight back the infection and are thus dependent on drugs to kill the parasites. Currently, there is no drug available specifically targeting cryptosporidiosis. Toxoplasmosis treatment is extremely difficult, with problematic complications associated with the few known effective drugs.
Apicomplexan parasites are a diverse group of protozoan parasites, several of which cause important human and animal diseases, such as malaria, cryptosporidiosis and toxoplasmosis. Many of these diseases are endemic in developing countries, which are impacted by the lack of cost-efficient treatments. Cryptosporidiosis and toxoplasmosis can also be life-threatening to immunocompromised individuals, e.g. those undergoing organ transplantation or chemotherapy and HIV/AIDS patients, who cannot fight back the infection and are thus dependent on drugs to kill the parasites. Currently, there is no drug available specifically targeting cryptosporidiosis. Toxoplasmosis treatment is extremely difficult, with problematic complications associated with the few known effective drugs.
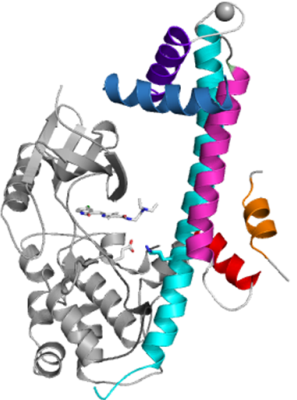
The group led by Dr. Raymond Hui at the Structural Genomics Consortium was created to identify and solve the structures of potential drug targets in apicomplexan genomes. In collaboration with Prof. David Sibley’s lab at the University of Washington in St. Louis, this group has just published their most recent findings in “Nature Structural & Molecular Biology”. In the article entitled “Structures of apicomplexan calcium-dependent protein kinases reveal mechanism of activation by calcium”, the authors describe the structures of several calcium dependent protein kinases (CDPKs) in both autoinhibited (apo) and activated (calcium-bound) conformations from the apicomplexan parasites Toxoplasma gondii and Cryptosporidium parvum.
In these parasites, calcium concentration plays an important role, controlling a number of essential pathways, including protein secretion, activation of motility, cell invasion and egress (exiting). This is mediated thorough a complex signalling pathway involving CDPKs that resemble CaMKs in the catalytic domain but are tightly regulated by an adjoining calcium sensing domain.
These newly published novel CDPK structures are the first to demonstrate the the interaction of the kinase and calcium binding domains. In addition other structures solved by the SGC reveal a surprising rearrangement of the regulatory calcium binding domain (also called C-terminal CDPK activation domain – CAD). In the apo form, CAD shows an unexpected long helix in the N terminus that blocks and inhibits the kinase domain in the same manner as seen in CaMKII. Calcium binding triggers the reorganization of the CAD into a highly intricate fold, leading to its relocation around the base of the kinase domain to a site remote from the substrate binding site. This large conformational change, which resembles the opening of a gate, constitutes a distinct mechanism in calcium signal-transduction pathways.
“Since humans don’t have any CDPKs, their particular structural features – such as the conformational changes seen here - can be explored to aid in the design of more potent and specific drugs,” said Dr. Hui. In addition to a unique regulatory mechanism, the ATP-binding site of two specific CDPKs described in the article, nameyly TgCDPK1 and CpCDPK1, also features a glycine in the gatekeeper position that creates a hydrophobic pocket not seen in other wild type kinases, including those present in humans. This can be exploited for design of selective inhibitors, as suggested in a companion article in the same issue of NSMB contributed by the Medical Structural Genomics of Pathogenic Protozoa Consortium headed by Dr. Wesley van Voorhis.
In the fight against infectious diseases, where effective treatments are scarce, each discovery that opens the possibility for the creation of a new therapy is welcomed. Structural biology continues to one of the main drivers in this field.
- Read the article in Nature Structural & Molecular Biology
- Read more about the structures: 3HZT, 3HX4, 3KU2, 3IGO
