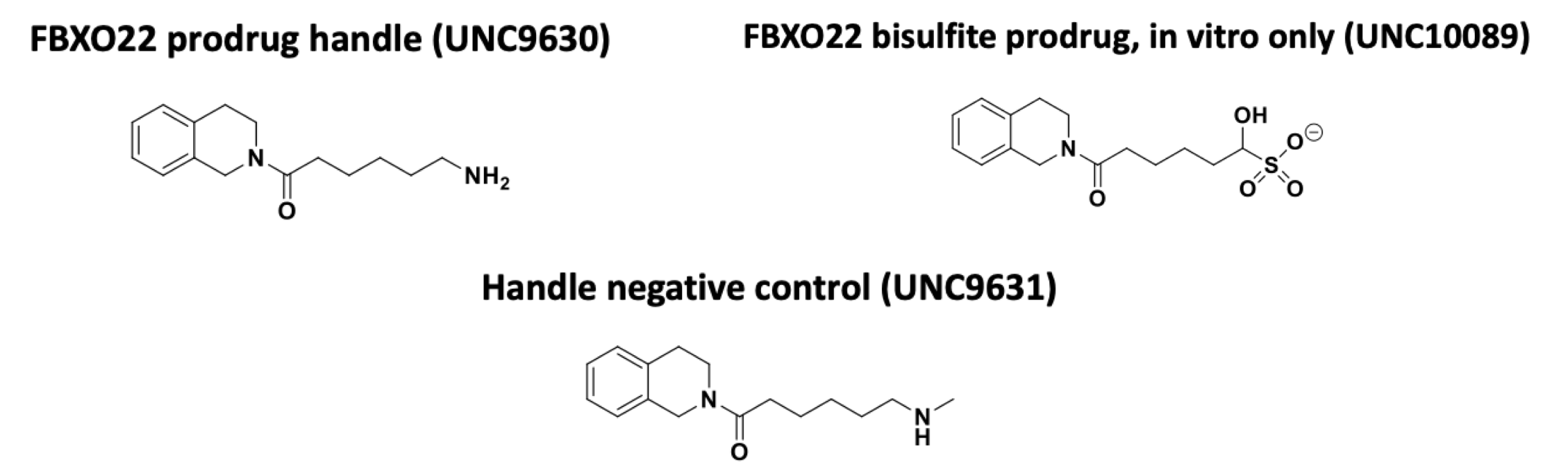
SGC in collaboration with Lindsey James’ lab at the University of North Carolina at Chapel Hill (UNC) has developed a chemical handle, UNC9630, for FBXO22.
This followed from the development of degraders of NSD2 (UNC8732) using the NSD2 PWWP1 ligand UNC6934, and the discovery of the mechanism of action via FBXO22.
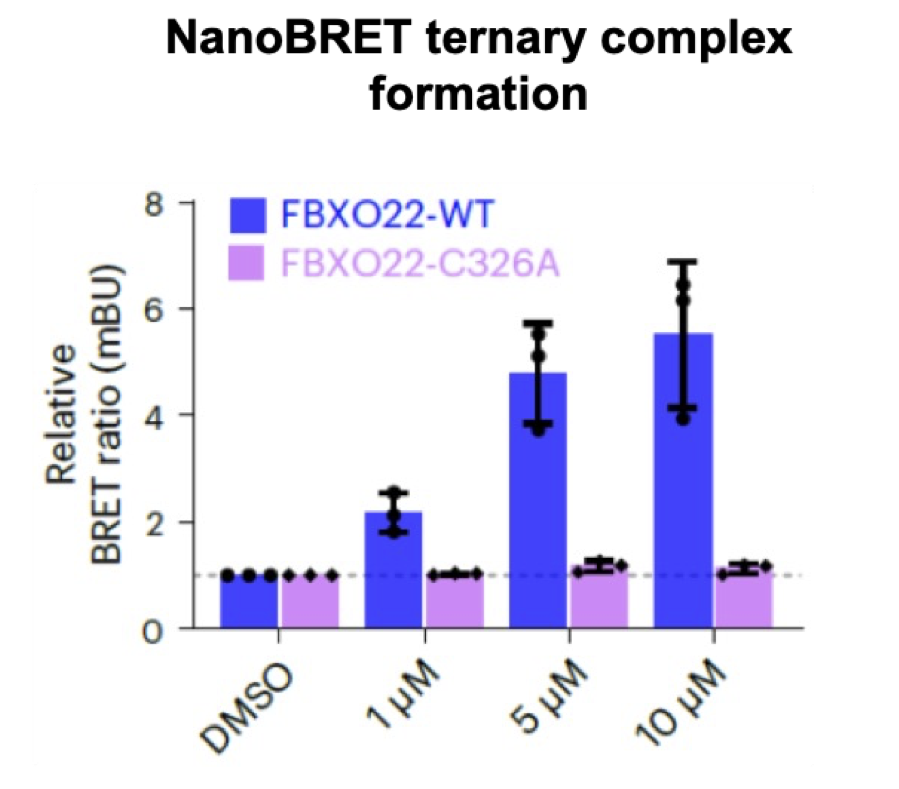
In cells, the primary amine is converted to the aldehyde which forms a covalent adduct with FBXO22 C326. For in vitro (cell-free) studies, the bisulfite adduct UNC10089 can be used as a prodrug for the aldehyde. The methylated secondary amine UNC9631 cannot be oxidized to the aldehyde and is a negative control.
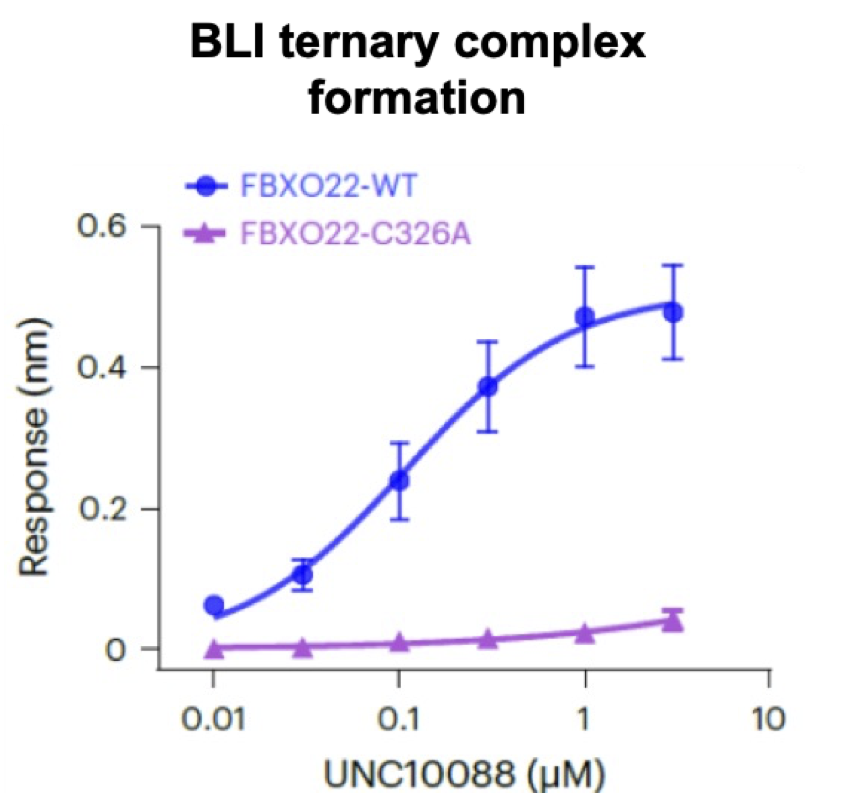
In cells, the primary amine is converted to the aldehyde which forms a covalent adduct with FBXO22 C326.