This probe is available from Tocris, Cayman Chemical, Sigma and opnMe.com.
| Probe | Negative control | |
 |
|  |
BI01383298 |
| BI01372674 |
SLC13A5 (NaCT, INDY) is a sodium-citrate co-transporter that is highly expressed in the liver. It is a member of the SLC13 family of which there are 4 other members. SLC13A5 transports citrate from the circulatory system into hepatocytes where it is used in the synthesis of sterols and fatty acids. SLC13A5 was first identified in Drosophila where the name I’m Not Dead Yet or INDY was coined. A study in 2000(1) showed that lower expression of SLC13A5 in Drosophila led to increased lifespan. Mouse models have demonstrated the potential of this protein as a target for obesity and diabetes with knockout mice protected from adiposity (2), reduced lipid concentrations in a siRNA study (3) and a substrate analogue used to lower blood glucose levels (4). More recently mutations in SLC13A5 have been linked to early-infantile epileptic encephalopathy (5) while silencing of the SLC13A5 gene inhibits proliferation of human hepatocarcinoma cells (6).
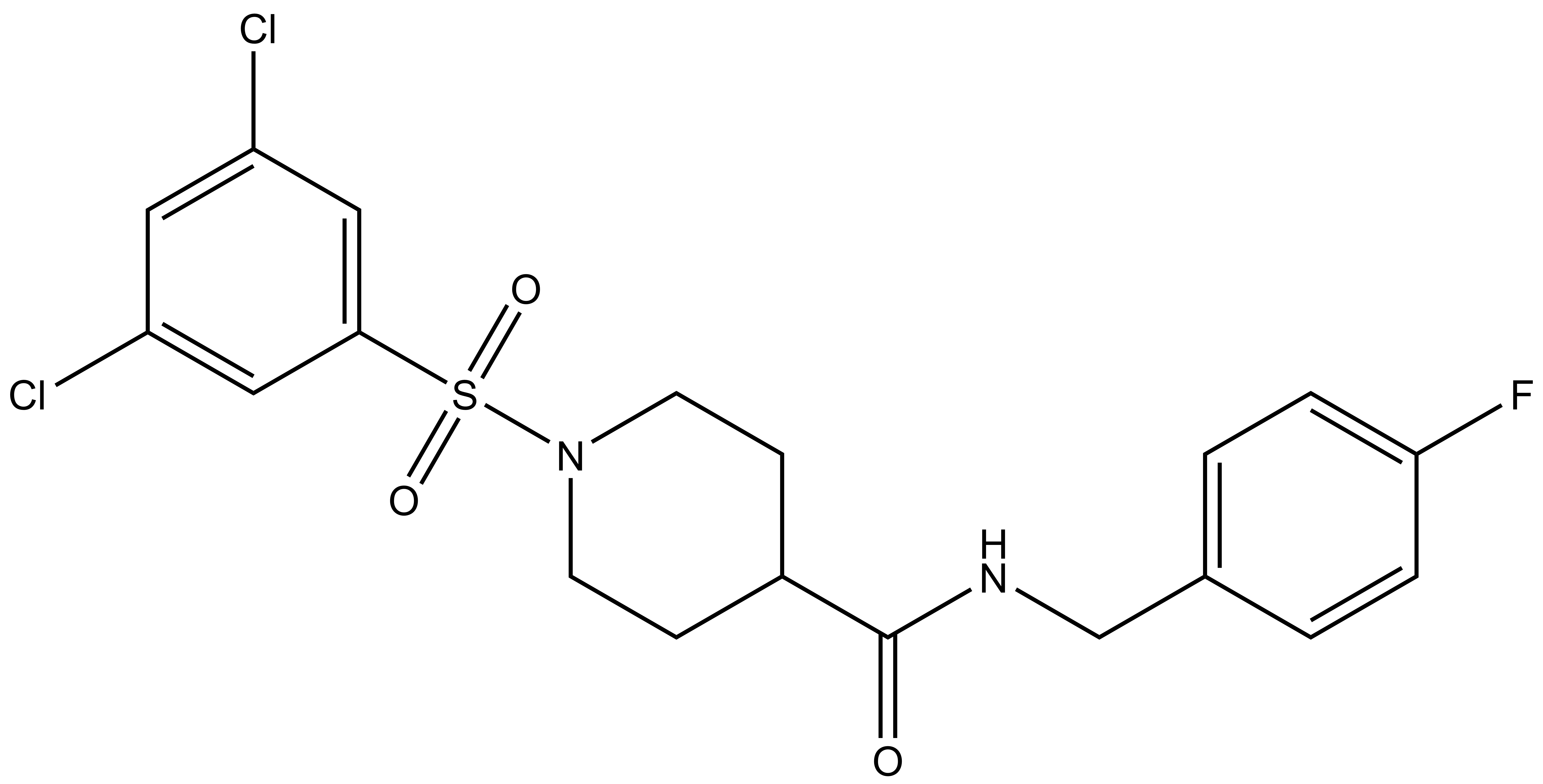
BI1383298 is a potent inhibitor of SLC13A5 which unlike previously published inhibitors (4,7) has no structural homology to the substrate (citrate). It is selective over other family members and other transporters. A chemically related negative control (BI01372674) is also provided.
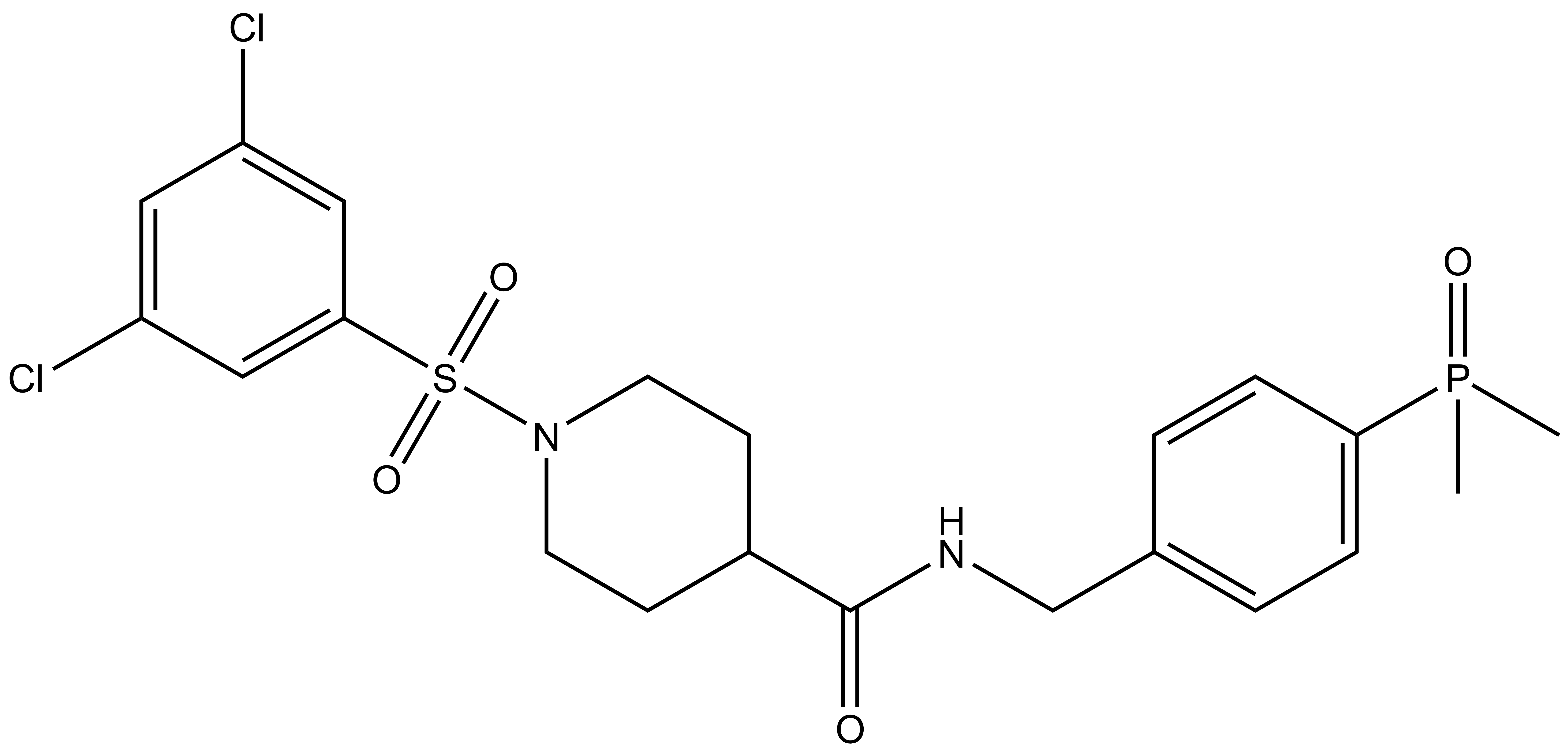
In addition to the chemical probe (BI01383298) we also include a negative control (BI01372674) which is chemically analogous to the probe molecule.
The probe molecule (BI01383298) and control (BI01372674) are soluble, after visual inspection, at 10 µM and 200µM in the presence of 0.1% DMSO in media and assay buffer.
Aliquots of stock Solutions were prepared as follows: 10mM BI01383298 in DMSO (Stored -20oC), 5mM BI01383298 in DMSO (Stored -20oC, need agitation upon thaw and gentle warming to 30oC).
BI01383298 but not BI01372674 demonstrates target engagement in vitro with purified human SLC13A5 protein as shown using a thermostabilisation assay with the Prometheus label-free system from Nanotemper (figure 2).
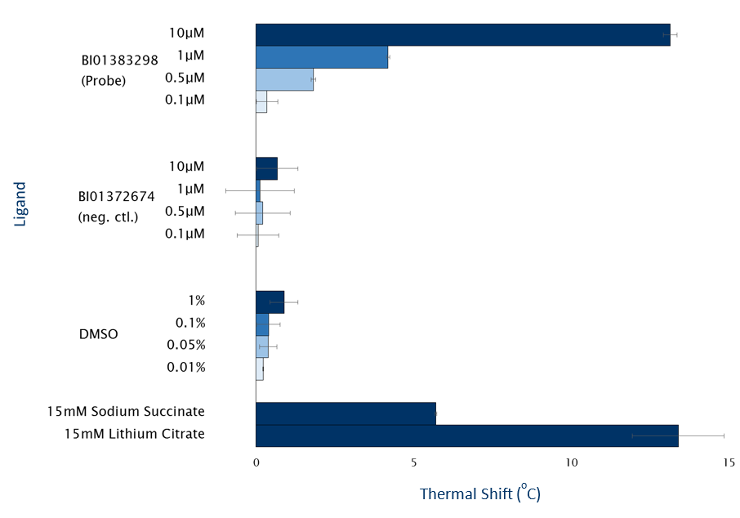
Figure 2: Thermostabilisation of human SLC13A5 by BI01383298 and BI01372674 between 0.1µM and 10µM. SLC13A5 assay concentration of 1µM. Data summarises 3 biological samples with between 4 and 8 replicates for each.
| Probe | Negative control | |
 |
|  |
BI01383298 |
| BI01372674 |
| Physical and chemical properties for BI01383298 | |
| Molecular weight | 444.0 |
| Molecular formula | C19H19Cl2FN2O3S |
| IUPAC name | (1-(3,5-dichloro-phenylsulfonyl)-piperidin-4-yl)-((4-fluoro-phenyl)-methylamino)-methanone |
| MollogP | 4.64 |
| PSA | 56.3 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 6 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 1 |
| Physical and chemical properties for BI1372674 (Negative Control) | |
| Molecular weight | 502.1 |
| Molecular formula | C21H25Cl2N2O4PS |
| IUPAC name | |
| MollogP | 3.54 |
| PSA | 69.5 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 7 |
| No. of hydrogen bond acceptors | 9 |
| No. of hydrogen bond donors | 1 |
SMILES:
BI01383298: FC(C=C1)=CC=C1CNC(C2CCN(S(C3=CC(Cl)=CC(Cl)=C3)(=O)=O)CC2)=O
BI1372674: ClC1=CC(Cl)=CC(S(=O)(N2CCC(CC2)C(NCC3=CC=C(C=C3)P(C)(C)=O)=O)=O)=C1
InChI:
BI01383298: InChI=1S/C19H19Cl2FN2O3S/c20-15-9-16(21)11-18(10-15)28(26,27)24-7-5-14(6-8-24)19(25)23-12-13-1-3-17(22)4-2-13/h1-4,9-11,14H,5-8,12H2,(H,23,25)
BI1372674: InChI=1S/C21H25Cl2N2O4PS/c1-30(2,27)19-5-3-15(4-6-19)14-24-21(26)16-7-9-25(10-8-16)31(28,29)20-12-17(22)11-18(23)13-20/h3-6,11-13,16H,7-10,14H2,1-2H3,(H,24,26)
InChIKey:
BI01383298: VUOYAALVGSMUHC-UHFFFAOYSA-N
BI1372674: GSNNWZZIEKEEQF-UHFFFAOYSA-N
| Solvent | BI01383298 | BI01372674 | |
| Solubility (200uM, 4% DMSO) | Assay Buffer*- 0hr | Soluble | Soluble |
| Assay Buffer*- 12hrs | Soluble | Soluble | |
| Freestyle Media- 0hr | Soluble | Soluble | |
| Freestyle Media- 12hr | Soluble | Soluble | |
| Water- 0hrs | ppt. observed | ppt. observed | |
| Solubility (10uM, 0.1% DMSO) | Assay Buffer*- 0hr | Soluble | Soluble |
| Assay Buffer*- 12hrs | Soluble | Soluble | |
| Freestyle Media- 0hr | Soluble | Soluble | |
| Freestyle Media- 12hr | Soluble | Soluble | |
| Water- 0hrs | Soluble | Soluble |
*50mM HEPES (pH 7.5), 200mM NaCl, 0.024% n-Dodecyl-β-D-Maltopyranoside, 0.0024% Cholesterol Hemisuccinate.
BI01383298 is more than 1000-fold selective (table 1) over the closest family members: human SLC13A2 / SLC13A3 that share physiological substrates citrate and succinate.
Table 1: Citrate uptake inhibition was measured for all citrate transporters and glycine uptake measured to GLYT2. Potency was assessed for the probe candidate and the negative control on uptake of 14C-citrate into cells over-expressing SLC13A5, SLC13A2, SLC13A3, mouse SLC13A5 and in HEPG2 cells.
| Substrate uptake inhibition IC50 [nM] | ||
| BI01383298 | BI01372674 | |
| HEK cells- hSLC13A5 | 56 | >100,000 |
| HepG2 cells | 24 | >100,000 |
| HEK cells- mSLC13A5 | >100,000 | 88,000 |
| HEK cells- hSLC13A2 | >100,000 | n.d. |
| HEK cells- hSLC13A3 | >100,000 | n.d. |
| HEK cells – GLYT2 | >100,000 | >100,000 |
Selectivity panel (% inhibition @ 10μM): 35/38 targets<50%; CB1(h): 78%; K(KOP): 81%; Na+channel: 52%
BI01383298 is a potent inhibitor of SLC13A5 with an apparent IC50 value of 56nM in HEK cells overexpressing SLC13A5 and 24nM in HepG2 cell expressing endogenous SLC13A5. BI01383298 was not found to inhibit citrate transport in HEK cells over-expressing mouse SLC13A5 and thus we do not recommend this compound for use in mouse models. BI01372674 was not found to inhibit citrate uptake in a HEPG2 model expressing endogenous levels of SLC13A5 or in HEK cellular models over-expressing human SLC13A5, mouse SLC13A5 or GLYT2.


Figure 1: Measured IC50 of hSLC13A5-mediated 14C-citrate in (A) overexpressed, HEK293-Flp-In-hSLC13A5 and (B) endogenous, HepG2 celluar models.