This probe (as hydrochloride) and its inactive control are available from Sigma (A-395 and A-395N) and Cayman (A-395).
| Probe | Negative control | |
 |
|  |
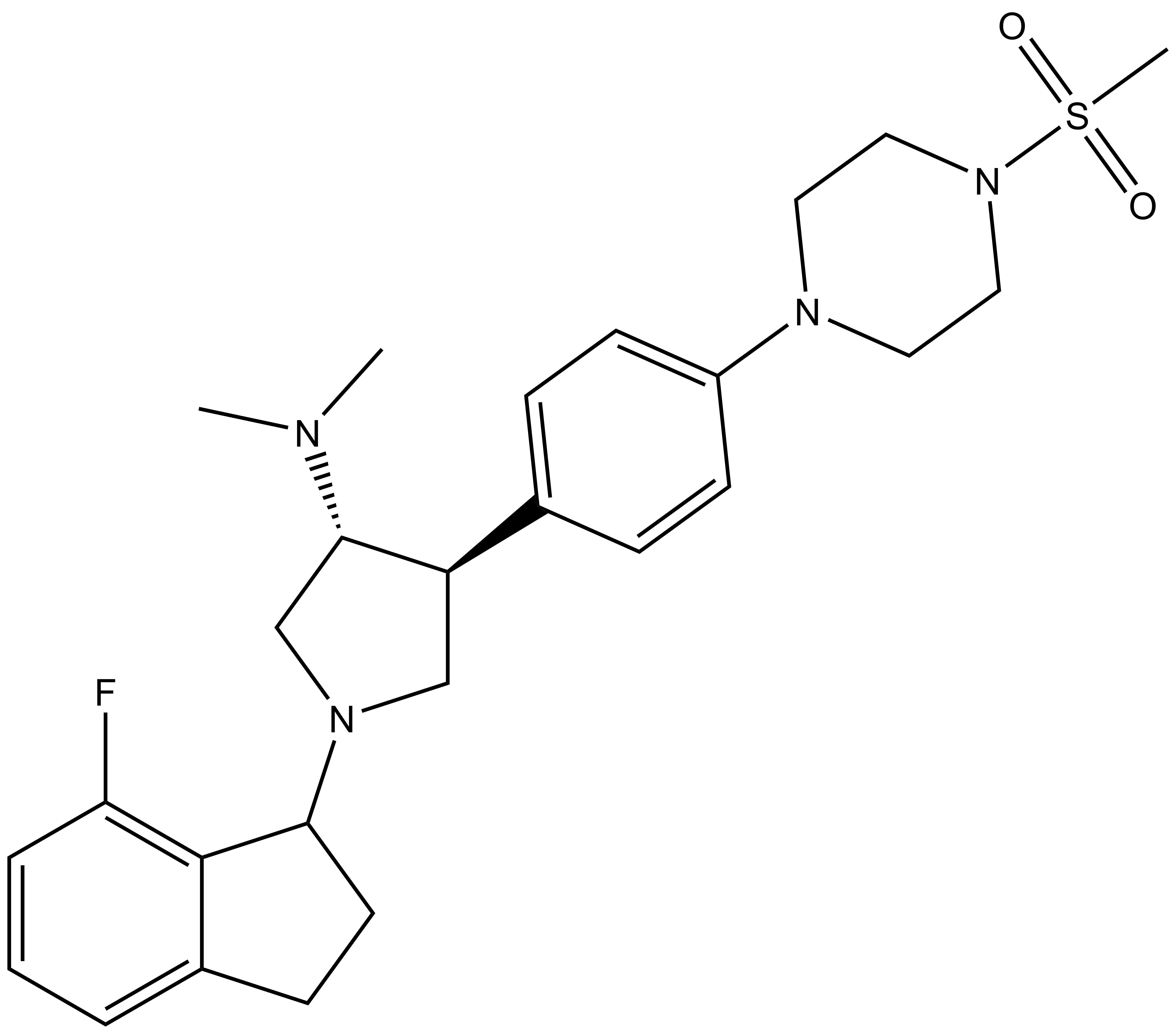
A-395 |
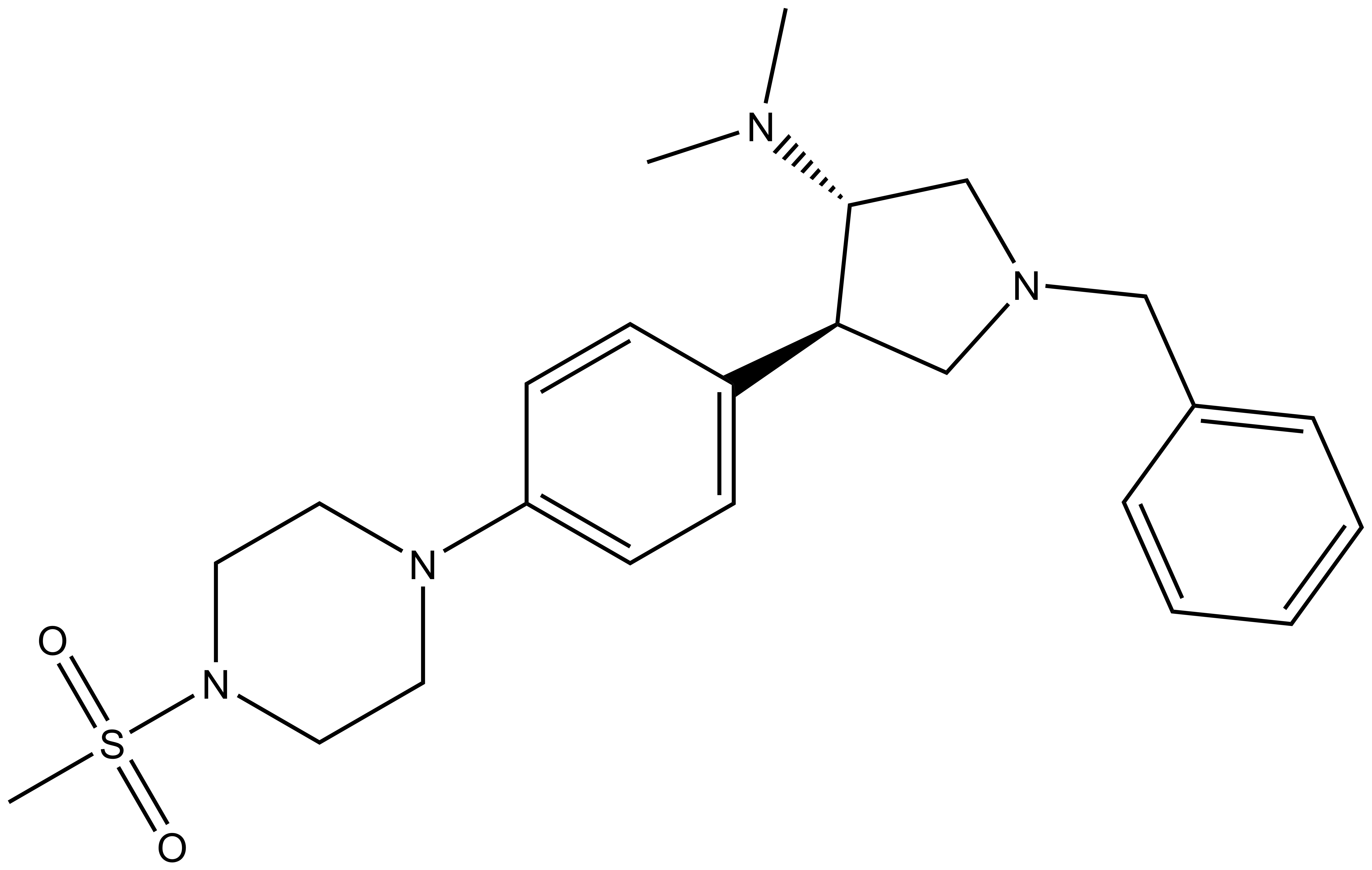
| A-395N |
A collaboration between Abbvie and the SGC has resulted in the discovery of A-395, the first potent and selective chemical probe for EED. The in vitro activity of A-395 includes potent binding to EED with Ki = 0.4 nM, inhibits the PRC2 complex with IC50 = 34 nM for methylation of H3K27 and greater than 100-fold selectivity over other histone methyltransferases and non-epigenetic targets. In cellular assays, A-395 inhibits the PRC2 complex (thus inhibiting the formation of H3K27me3) with IC50 = 90 nM (RD rhabdoid tumor cell line; 3 days).
A closely related compound, A-395N, exhibits no activity in the biochemical and cellular assays, and is an ideal control compound for cellular studies.
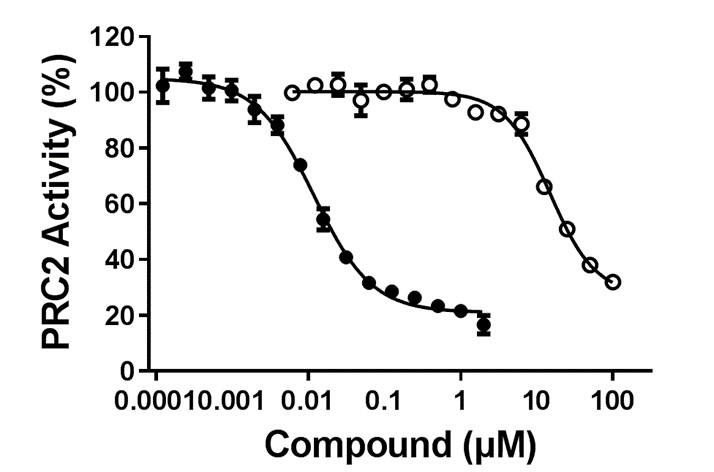
A-395 potently inhibited the formation of H3K27me3 (via antagonizing EED in the trimeric PRC2 complex (EZH2:EED:SUZ12)) with IC50 = 34 ± 2 nM (Hill Slope = 0.7)
| Probe | Negative control | |
 |
|  |
A-395 |
| A-395N |
| Physical and chemical properties for A-395 | |
| Molecular weight | 486.2 |
| Molecular formula | C26H35FN4O2S |
| IUPAC name | 1-(5-fluoro-bicyclo[4.3.0]nona-1,3,5-trien-7-yl)-N,N-dimethyl-4-(4-(4-methylsulfonyl-piperazin-1-yl)-phenyl)-pyrrolidin-3-amine |
| MollogP | 4.425 |
| PSA | 39.69 |
| No. of chiral centres | 3 |
| No. of rotatable bonds | 5 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 0 |
| Physical and chemical properties for A-395N | |
| Molecular weight | 442.2
|
| Molecular formula | C24H34N4O2S |
| IUPAC name | 1-benzyl-N,N-dimethyl-4-(4-(4-methylsulfonyl-piperazin-1-yl)-phenyl)-pyrrolidin-3-amine |
| MollogP | 3.158 |
| PSA | 40.58 |
| No. of chiral centres | 2 |
| No. of rotatable bonds | 6 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 0 |
Selectivity of A-395 against 32 methyltransferase enzymes
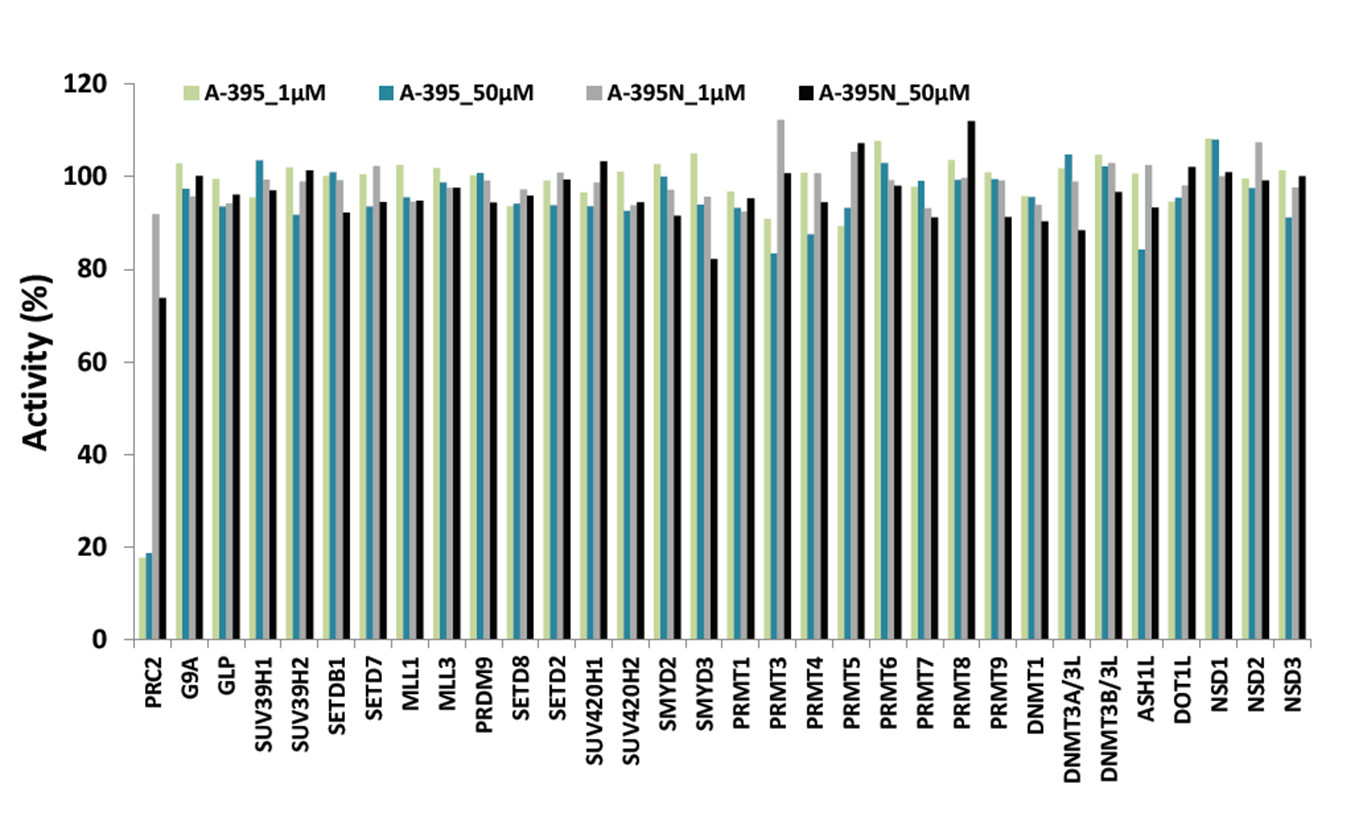
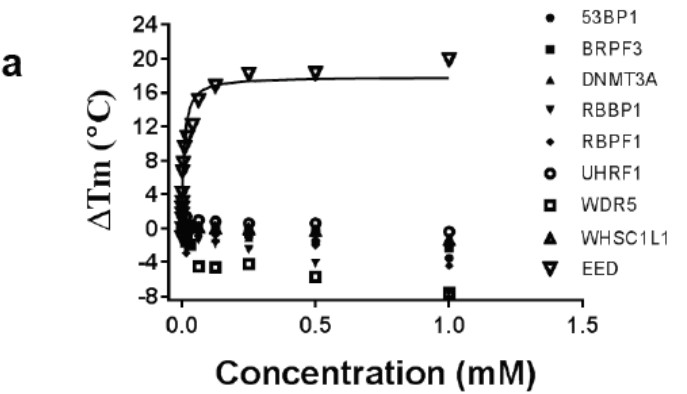
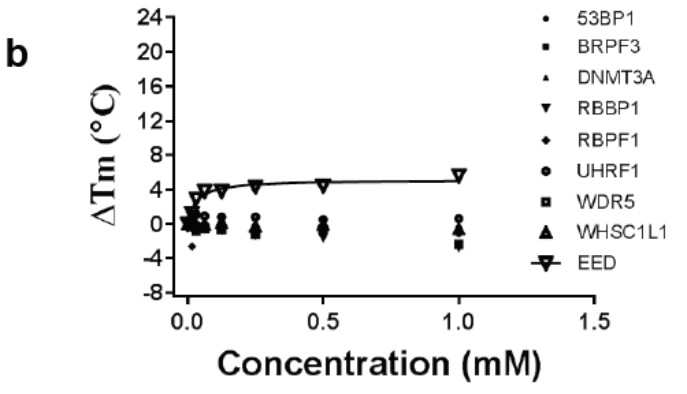
Selectivity of a) A-395 and b) A-395N relative to epigenetic readers using a thermal shift assay
 |  |
A-395 inhibits the activity of the PRC2 complex in cells – high-content screening assay of RD Rhabdoid tumor cell line treated for 72 hours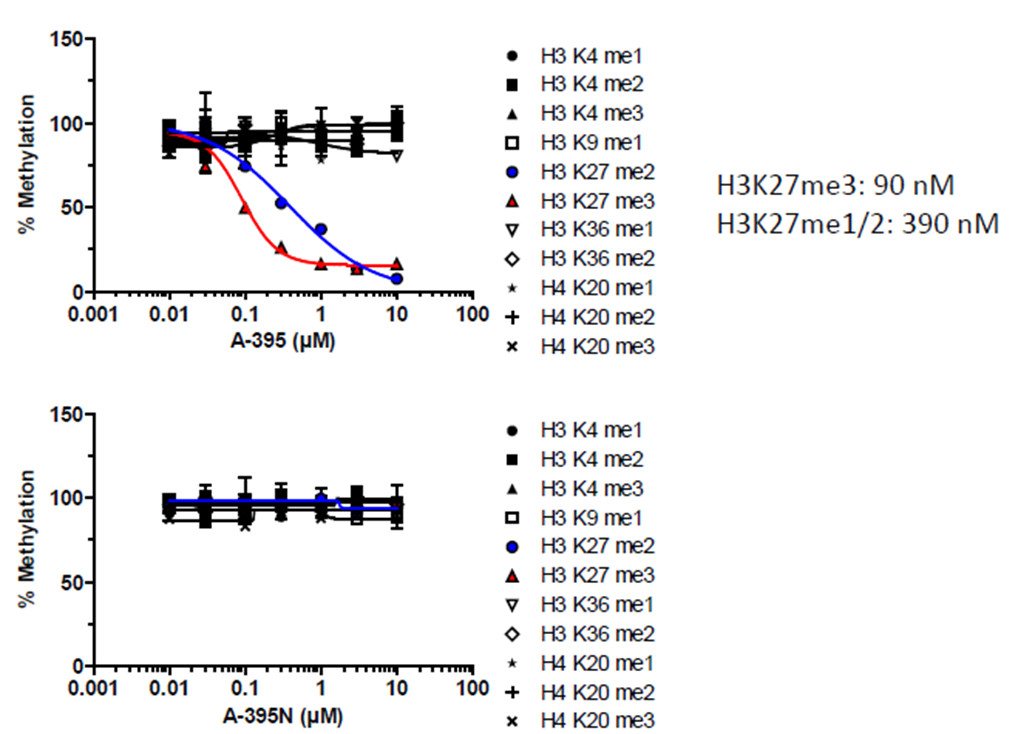
Main features