This probe is available from Tocris, Cayman Chemical and Sigma.
Its negative control (SGC-AAK1-1N) is available for purchase from Tocris and Sigma.
| Probe | Negative control | |
 |
|  |
SGC-AAK1-1 |
| SGC-AAK1-1N |
AAK1 (adaptor protein 2-associated kinase) and BMP2K/BIKE (BMP-2 inducible kinase) comprise half of the numb-associated kinase (NAK) family, which also includes cyclin G associated kinase (GAK) and STK16/MPSK1 (serine/threonine kinase 16/myristoylated and palmitoylated serine/threonine kinase 1).1
AAK1 is a 104 kDa serine/threonine kinase with broad tissue expression. Within the cell AAK1 localizes to the cell membrane and cytoplasm.2 AAK1 is involved in clathrin-mediated endocytosis (CME), both by direct binding to clathrin and by phosphorylation of the medium subunit of AP-2 (adaptor protein 2).3-5 In this manner, AAK1 has been identified as a negative regulator of Wnt signaling via mediation of LRP6 internalization. Studies have implicated multiple roles for AAK1 in influencing Notch signaling, including priming and redistribution of Numb as well as Notch activation.6-7
Relatively less is known about the highly understudied kinase BIKE. BIKE is broadly expressed, and in the cell, it localizes to nuclear speckles.2 BIKE was originally identified as its expression was observed to increase upon bone morphogenic protein (BMP-2)-induced differentiation of a prechondroblastic cell line.8 The same study provided evidence for BIKE having an important regulatory role in attenuating the program of osteoblast differentiation. Proteomic studies identified BIKE as a clathrin vesicle-associated protein and have also identified interaction between BIKE and Numb.9-10
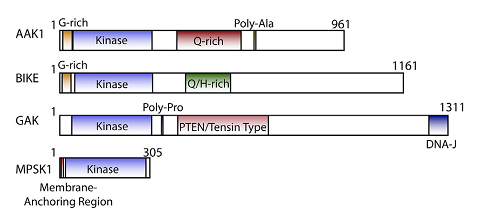
NAK family domain structures
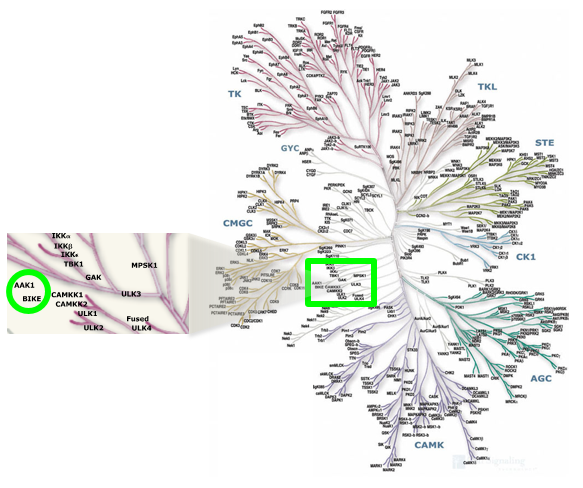
Location of AAK1 and BIKE on kinome tree
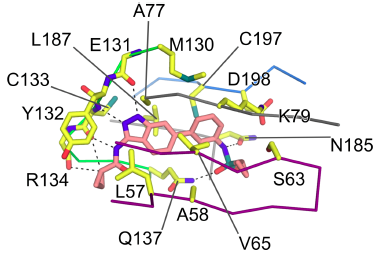
Snapshot of crystal structure of acylaminoindazole bound to BIKE
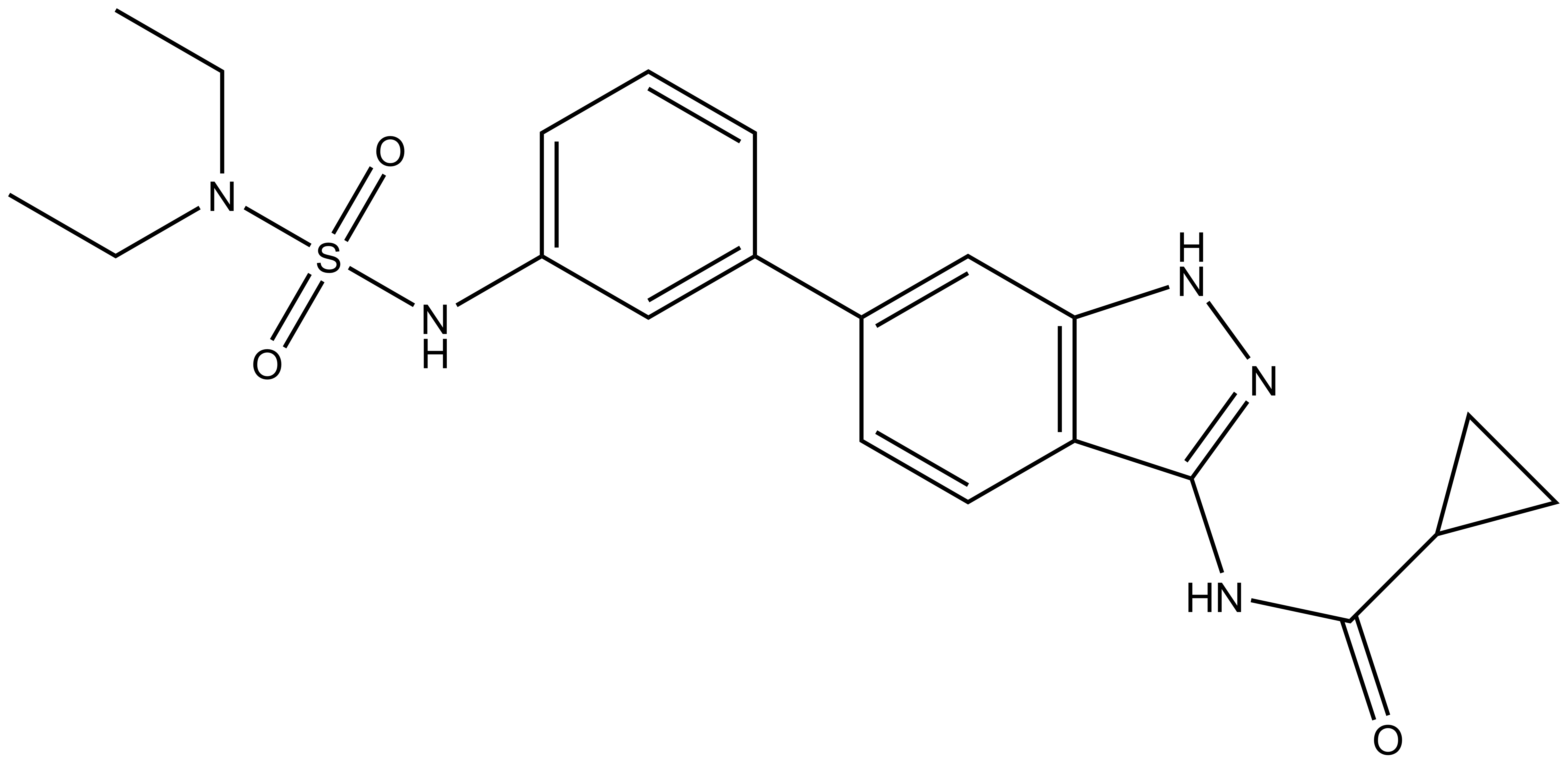
SGC-AAK1-1 is a chemical probe for AAK1 and BIKE that potently targets the ATP-binding site (AAK1 Ki = 9.1 nM; BIKE Ki = 17 nM). Regarding kinase selectivity, only three kinases were observed to bind SGC-AAK1-1 within 30-fold of the KD of AAK1 in a KINOMEscan assay (DiscoverX) at 1 μM followed by KD determinations: RIOK1 (KD = 72 nM), RIOK3 (KD = 290 nM), and PIP5K1C (KD = 260 nM). In a live cell NanoBRET assay (Promega) SGC-AAK1-1 has potency for ectopically expressed full-length AAK1- and BIKE-Nluc fusion proteins (AAK1 IC50 = 230 nM; BIKE IC50 = 1.5 μM).
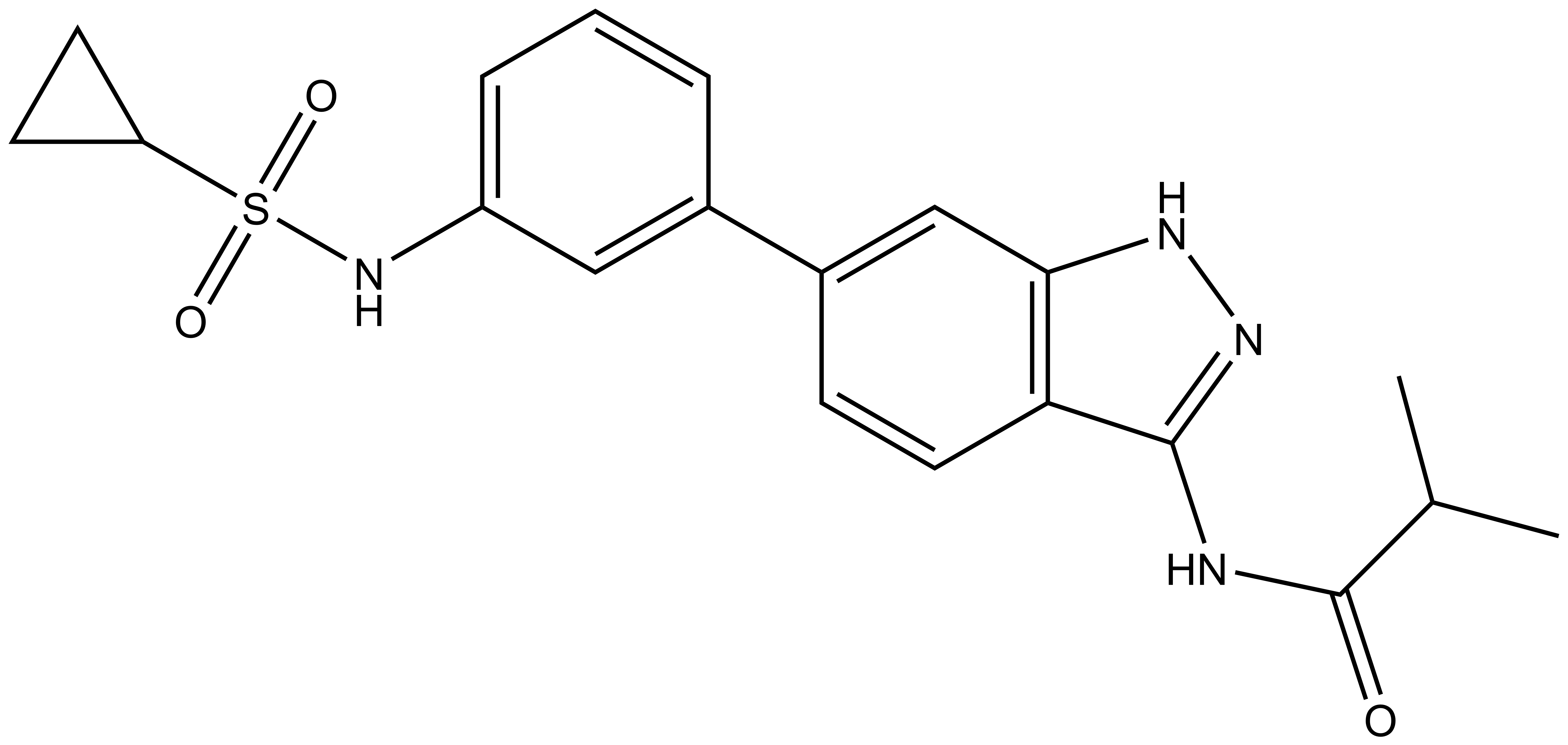
A chemically related negative control compound, SGC-AAK1-1N, is provided.
| Probe | Negative control | |
 |
|  |
SGC-AAK1-1 |
| SGC-AAK1-1N |
| Physical and chemical properties for BAY-876 | |
| Molecular weight | 427.52 |
| Molecular formula | C21H25N5O3S |
| IUPAC name | N-(6-(3-((N,N-diethylsulfamoyl)amino)phenyl)-1H-indazol-3-yl)cyclopropanecarboxamide |
| MollogP | 4.185 |
| PSA | 89.47 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 9 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 3 |
| Storage | -20 °C as DMSO stock |
| Dissolution | Soluble in DMSO at least up to 10 mM |
| Physical and chemical properties for BAY-588 (Negative Control) | |
| Molecular weight | 398.48 |
| Molecular formula | C20H22N4O3S |
| IUPAC name | N-(6-(3-(cyclopropanesulfonamido)phenyl)-1H-indazol-3-yl)isobutyramide |
| MollogP | 3.381 |
| PSA | 87.62 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 7 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 3 |
| Storage | -20 °C as DMSO stock |
| Dissolution | Soluble in DMSO at least up to 10 mM |
SMILES:
SGC-AAK1-1: O=C(NC1=NNC2=C1C=CC(C3=CC(NS(N(CC)CC)(=O)=O)=CC=C3)=C2)C4CC4
SGC-AAK1-1N: O=C(NC1=NNC2=C1C=CC(C3=CC(NS(C4CC4)(=O)=O)=CC=C3)=C2)C(C)C
InChI:
SGC-AAK1-1: InChI=1S/C21H25N5O3S/c1-3-26(4-2)30(28,29)25-17-7-5-6-15(12-17)16-10-11-18-19(13-16)23-24-20(18)22-21(27)14-8-9-14/h5-7,10-14,25H,3-4,8-9H2,1-2H3,(H2,22,23,24,27)
SGC-AAK1-1N: InChI=1S/C20H22N4O3S/c1-12(2)20(25)21-19-17-9-6-14(11-18(17)22-23-19)13-4-3-5-15(10-13)24-28(26,27)16-7-8-16/h3-6,9-12,16,24H,7-8H2,1-2H3,(H2,21,22,23,25)
InChIKey:
SGC-AAK1-1: UCBIQZUJJSVQHL-UHFFFAOYSA-N
SGC-AAK1-1N: RAIAORGFMNXPOV-UHFFFAOYSA-N