This probe is available from Cayman Chemical, Tocris and Sigma
| Probe |
 |
UNC0642 |
G9a (EHMT2) and GLP (EHMT1) catalyze the mono and dimethylation of lysine 9 of histone 3 (H3K9) and other non-histone substrates such as p53 and WIZ.
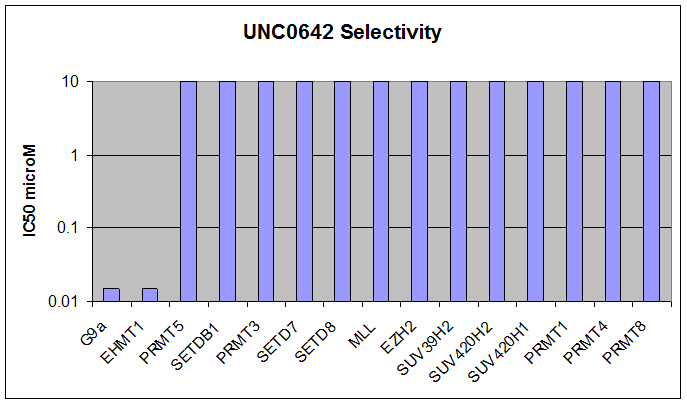
Here we present UNC0642, a G9a/GLP chemical probe with improved PK properties relative to UNC0638. UNC0642 exhibits an in vitro IC50 <15 nM with selectivity > 100-fold over 13 other HMTs and selected representatives of kinases, ion channels, 7TMs, and other epigenetic proteins.
In cells, UNC0642 results in a potent reduction of H3K9me2 in MDA MB231 cells with IC50 = 106 nM.
2-(4,4-difluoropiperidin-1-yl)-6-methoxy-N-[1-(propan-2-yl)piperidin-4-yl]-7-[3-(pyrrolidin-1-yl)propoxy]quinazolin-4-amine
Click here to download SDF file
| Physical and chemical properties | |
|---|---|
| Molecular weight | 546.3 |
| Molecular formula | C29H44F2N6O2 |
| IUPAC name | 2-(4,4-difluoropiperidin-1-yl)-6-methoxy-N-[1-(propan-2-yl)piperidin-4-yl]-7-[3-(pyrrolidin-1-yl)propoxy]quinazolin-4-amine |
| clogP | 5.96 |
| PSA | 54.6 A |
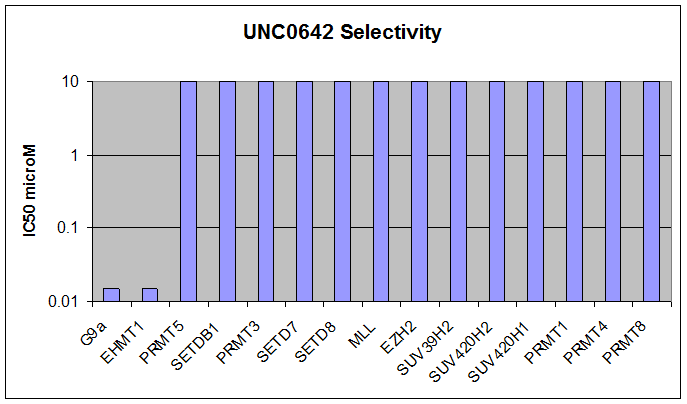
Below we show UNC0642 is equipotent to UNC0638 in the G9a in vitro assay:
G9a: Ki = 4 ± 2 (nM)
Competitive with peptide substrate
Non-competitive with SAM cofactor
Similar potency as UNC0638 (Ki = 3 nM)
In addition, UNC0642 has been shown to be inactive versus 50 kinases at 10 uM and has a similar GPCR selectivity as UNC0638.


a i.p. administration of a single 5 mg/kg dose in male Swiss Albino mice. 8 time points and 3 animals per time point
MDA-MB231 cells were cultured in RPMI with 10% FBS and MCF7 cells cultured in DMEM with 10% FBS.
Cells were grown in the presence or absence of inhibitors for stated amount of time. The media was removed and replaced with DMEM 10% FBS without phenol red supplemented with 1mg/ml of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) and incubated for 1-2h. Live cells reduce yellow MTT to purple formazan. The resulting formazan was solubilized in acidified isopropanol and 1% Triton and absorbance measured at 570nm, corrected for 650nm background.
Cells were grown in 96-well plates in the presence of inhibitors as stated in figures. Media was removed by flicking and 2% formaldehyde in PBS added for 15min. After five washes with 0.1% Triton X100 in PBS, cells were blocked for 1h with 1% BSA in PBS. Three out of four replicates were exposed to primary H3K9m2 antibody, Abcam #1220 at 1/800 dilution in 1% BSA, PBS for 2h. One replicate was reserved for background control. The wells were washed five times with 0.1% Tween 20 in PBS, then secondary IR800 conjugated antibody (LiCor) and DNA-intercalating dye, DRAQ5 (LiCor) added for 1h. After 5 washes with 0.1% Tween 20 in PBS, the plates were read on Odyssey (LiCor) scanner at 800nm (H3K9m2 signal; 764nm excitation) and 700nm (DRAQ5 signal; 683nm excitation). Fluorescence intensity was quantified, normalized to background and DRAQ5 signal expressed as percentage of control.
\
Discovery of an in Vivo Chemical Probe of the Lysine Methyltransferases G9a and GLP; Feng Liu, Dalia Barsyte-Lovejoy, Fengling Li, Yan Xiong, Victoria Korboukh, Xi-Ping Huang, Abdellah Allali-Hassani, William P. Janzen, Bryan L. Roth, Stephen V. Frye, Cheryl H. Arrowsmith, Peter J. Brown, Masoud Vedadi, and Jian Jin; J. Med. Chem., 2013, 56 (21), pp 8931–8942.