This probe is available from Sigma and Cayman Chemical.
| Probe | Negative control | |
 |
|  |
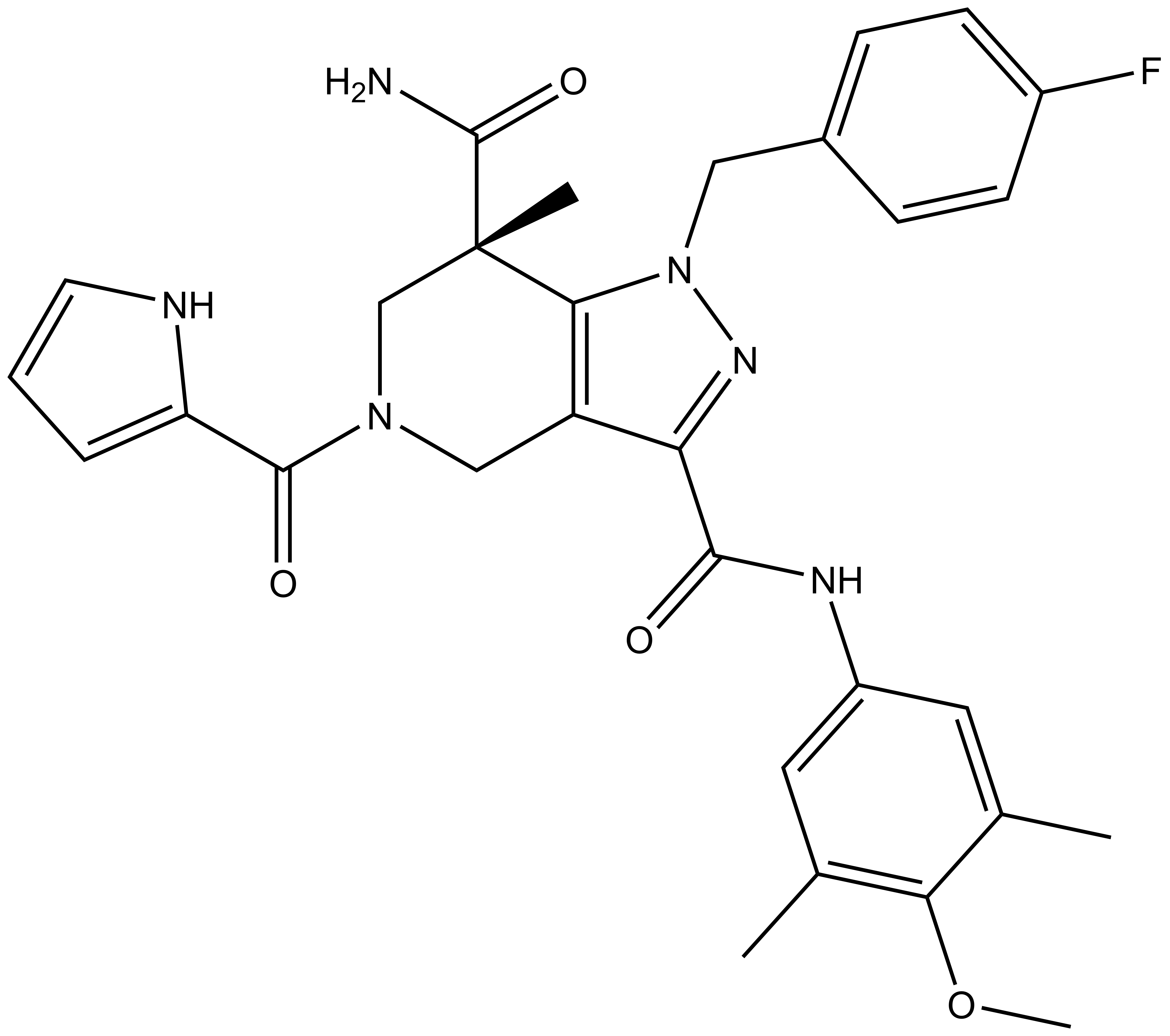
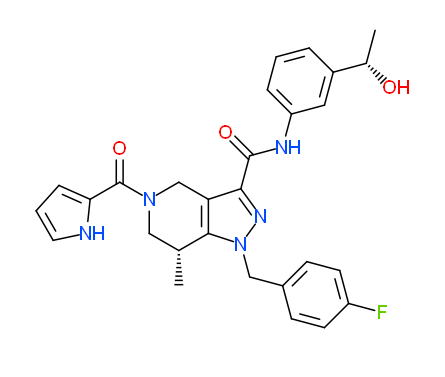
GSK864 |
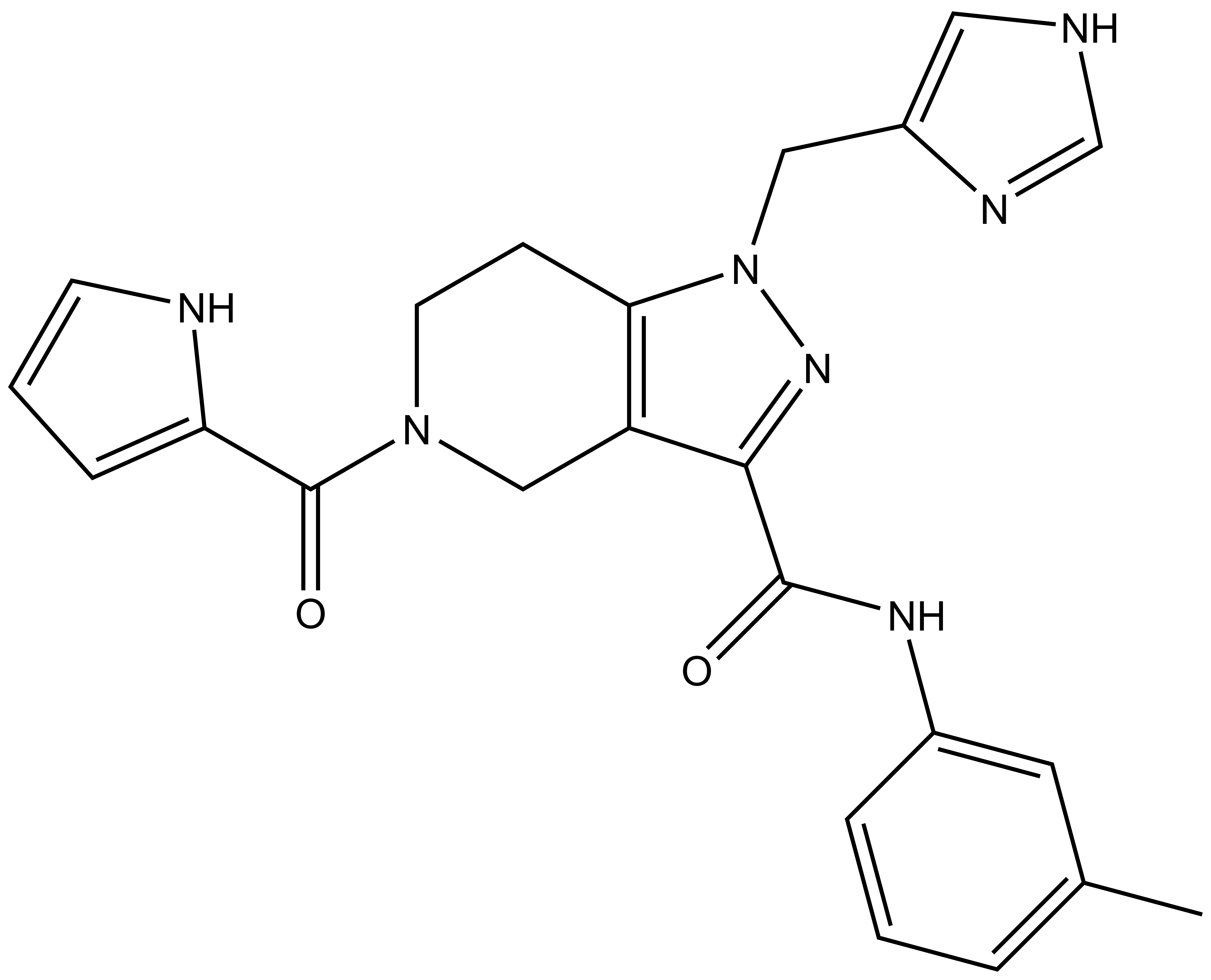
| GSK990 |
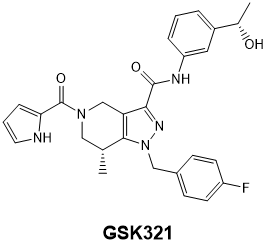
GlaxoSmithKline has developed GSK864 [1], a potent and selective inhibitor of mutant IDH1, and has made this available as an SGC chemical probe. GSK864 inhibits IDH1 mutants R132C/R132H/R132G with IC50 values of 9/15/17 nM, respectively, and is moderately selective over wild-type IDH1 and IDH2 mutants/wild-type. Treatment of R132C IDH1 mutant HT-1080 cells for 24 hours with GSK864 results in a dose-dependent reduction of 2-hydroxyglutarate (2-HG), which is not observed with GSK990, a structurally similar compound which is inactive as an IDH1 inhibitor. GSK864 has been shown to be selective in vitro for IDH1 over other classes of proteins (7TMs, ion channels, kinases) and chemoproteomic studies with GSK321, an analog of GSK864, confirm selective binding of IDH1 by this chemical series. GSK864 has a pharmacokinetic profile suitable for in vivo studies.
Chemoproteomics: the selectivity of GSK864 for IDH1 was illustrated by conducting a chemoproteomics experiment with a closely related analog, GSK321 [1]. GSK321 was functionalized so that it could be immobilized to NHS-activated Sepharose beads which were then incubated with a lysate (protein concentration 5 mg/mL) from HT-1080 cells. The experimental plan included using GSK990 and vehicle as control bait. The proteins were eluted from the beads and then subjected to in-gel digestion and labelling with TMT reagents. LC/MS/MS identified over 300 proteins of which only one, IDH1 had IC50 < 200 nM.
| Probe | Negative control | |
 |
|  |
GSK864 |
| GSK990 |
| Physical and chemical properties for GSK864 | |
| Molecular weight | 560.3 |
| Molecular formula | C30H33FN6O4 |
| MollogP | 3.162 |
| PSA | 98.62 |
| No. of chiral centres | 1 |
| No. of rotatable bonds | 10 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 4 |
| Physical and chemical properties for GSK990 (Negative Control) | |
| Molecular weight | 429.2 |
| Molecular formula | C23H23N7O2 |
| IUPAC name | (7-((1H-imidazol-4-yl)-methyl)-9-((3-methyl-phenylamino)-formyl)-3,7,8-triaza-bicyclo[4.3.0]nona-1(6),8-dien-3-yl)-(1H-pyrrol-2-yl)-methanone |
| MollogP | 1.316 |
| PSA | 85.96 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 7 |
| No. of hydrogen bond acceptors | 6 |
| No. of hydrogen bond donors | 3 |
|
| Physical and chemical properties for GSK990 (Negative Control) | |
| Molecular weight | 501.2 |
| Molecular formula | C28H28FN5O3 |
| IUPAC name | (7-(4-fluoro-benzyl)-9-((3-(1-hydroxy-ethyl)-phenylamino)-formyl)-5-methyl-3,7,8-triaza-bicyclo[4.3.0]nona-1(6),8-dien-3-yl)-(1H-pyrrol-2-yl)-methanone |
| MollogP | 3.285 |
| PSA | 79.77 |
| No. of chiral centres | 2 |
| No. of rotatable bonds | 8 |
| No. of hydrogen bond acceptors | 6 |
| No. of hydrogen bond donors | 3 |