| Probe | Negative control | |
 |
|  |
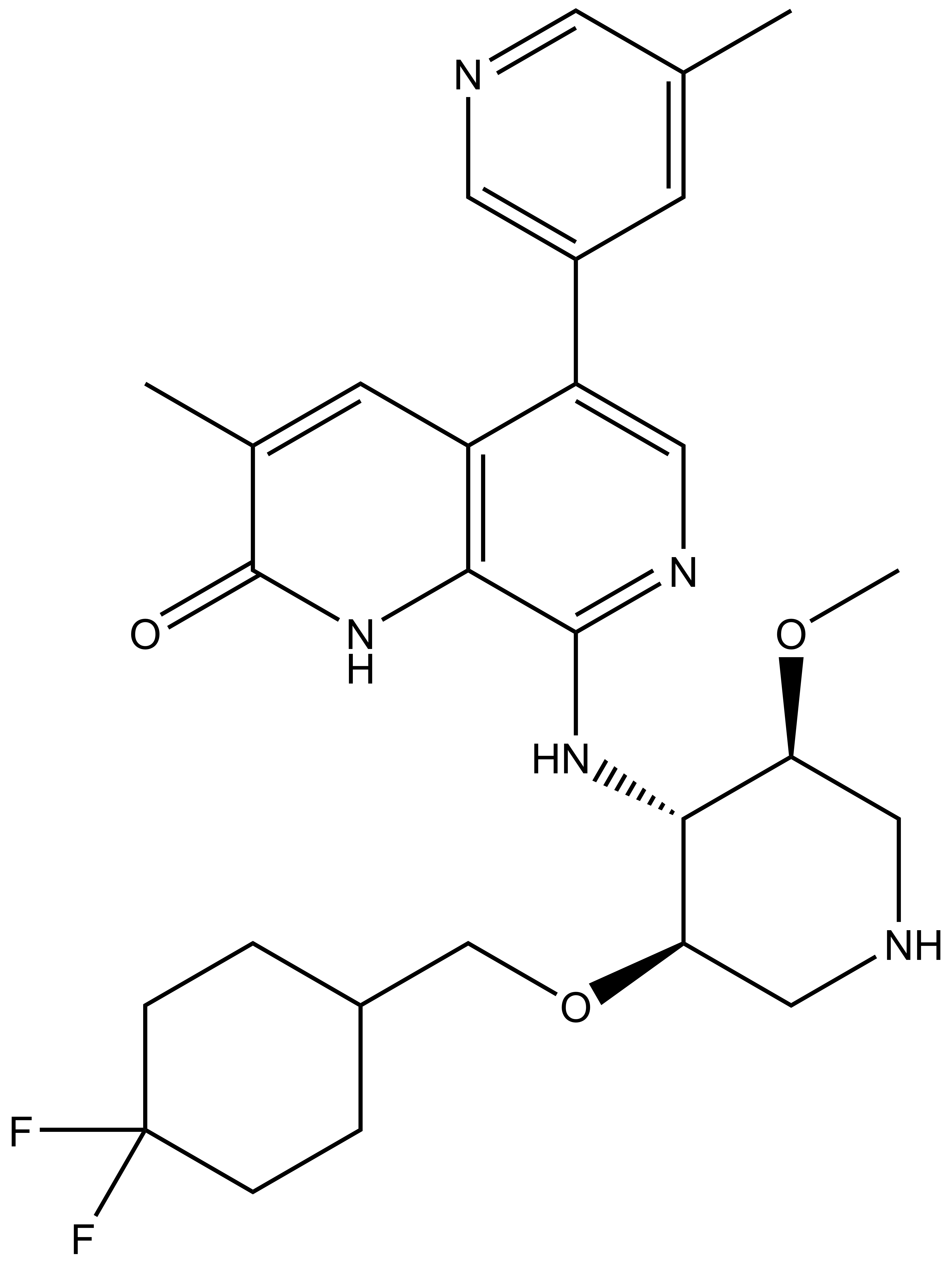
GSK8814 |
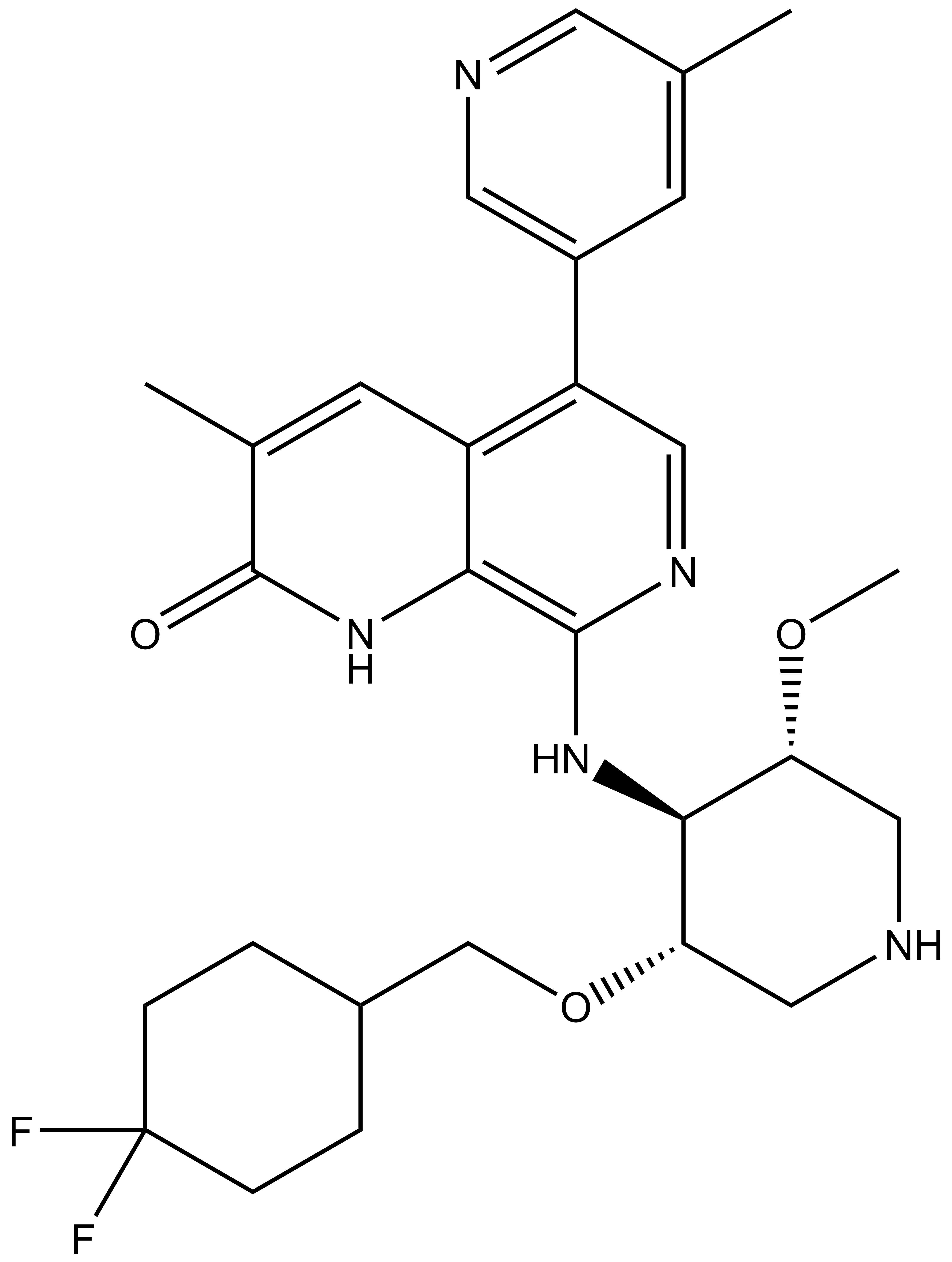
| GSK8815 |
ATAD2 and ATAD2B are chromatin remodelling factors and modulate the expression of multiple tumour cell growth factors. ATAD2 overexpression correlates with poor outcomes in several cancers. The bromodomain module of ATAD2 has been shown to be essential for its association with acetylated chromatin and presumable function.
GSK8814 is a chemical probe for the ATAD2/2B bromodomain, with a binding constant pKd=8.1 as measured by ITC (Bamborough et al, 2016). GSK8814 displaces acetylated H4 peptide from the ATAD2 bromodomain with pIC50 =7.3 and is also active in BROMOscan with pKi=8.9. It is more than 100 fold selective over all other bromodomains in the BROMOscan. Importantly, it is more than 1000 fold selective over BRD4. GSK8814 shows cellular target engagement with an EC50 of 2 µM in a NanoBRET assay evaluating the interaction of the NanoLuc-ATAD2 bromodomain with histone H3.3-HaloTag.
GSK8815 is a companion control compound with strongly reduced potency against ATAD2 (pKd=5.5).p>
| Probe | Negative control | |
 |
|  |
GSK8814 |
| GSK8815 |
| Physical and chemical properties for GSK8814 | |
| Molecular weight | 527.3 |
| Molecular formula | C28H35F2N5O3 |
| IUPAC name | 10-(3-((4,4-difluoro-cyclohexyl)-methoxy)-5-methoxy-piperidin-4-ylamino)-4-methyl-7-(5-methyl-pyridin-3-yl)-2,9-diaza-bicyclo[4.4.0]deca-1(10),4,6,8-tetraen-3-one |
| MollogP | 3.212 |
| PSA | 78.42 |
| No. of chiral centres | 3 |
| No. of rotatable bonds | 7 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 3 |
| Physical and chemical properties for GSK8815 (Negative Control) | |
| Molecular weight | 527.3 |
| Molecular formula | C28H35F2N5O3 |
| IUPAC name | 10-(3-((4,4-difluoro-cyclohexyl)-methoxy)-5-methoxy-piperidin-4-ylamino)-4-methyl-7-(5-methyl-pyridin-3-yl)-2,9-diaza-bicyclo[4.4.0]deca-1(10),4,6,8-tetraen-3-one |
| MollogP | 3.212 |
| PSA | 78.42 |
| No. of chiral centres | 3 |
| No. of rotatable bonds | 7 |
| No. of hydrogen bond acceptors | 7 |
| No. of hydrogen bond donors | 3 |
Bamborough P, Chung CW, Demont EH, Furze RC, Bannister AJ, Che KH, Diallo H, Douault C, Grandi P, Kouzarides T, Michon AM, Mitchell DJ, Prinjha RK, Rau C, Robson S, Sheppard RJ, Upton R, Watson RJ. A Chemical Probe for the ATAD2 Bromodomain. Angew Chem Int Ed Engl. 2016, 55(38):11382-6.