This probe is available from Cayman Chemical and Sigma.
| Probe | Negative control | |
 |
|  |
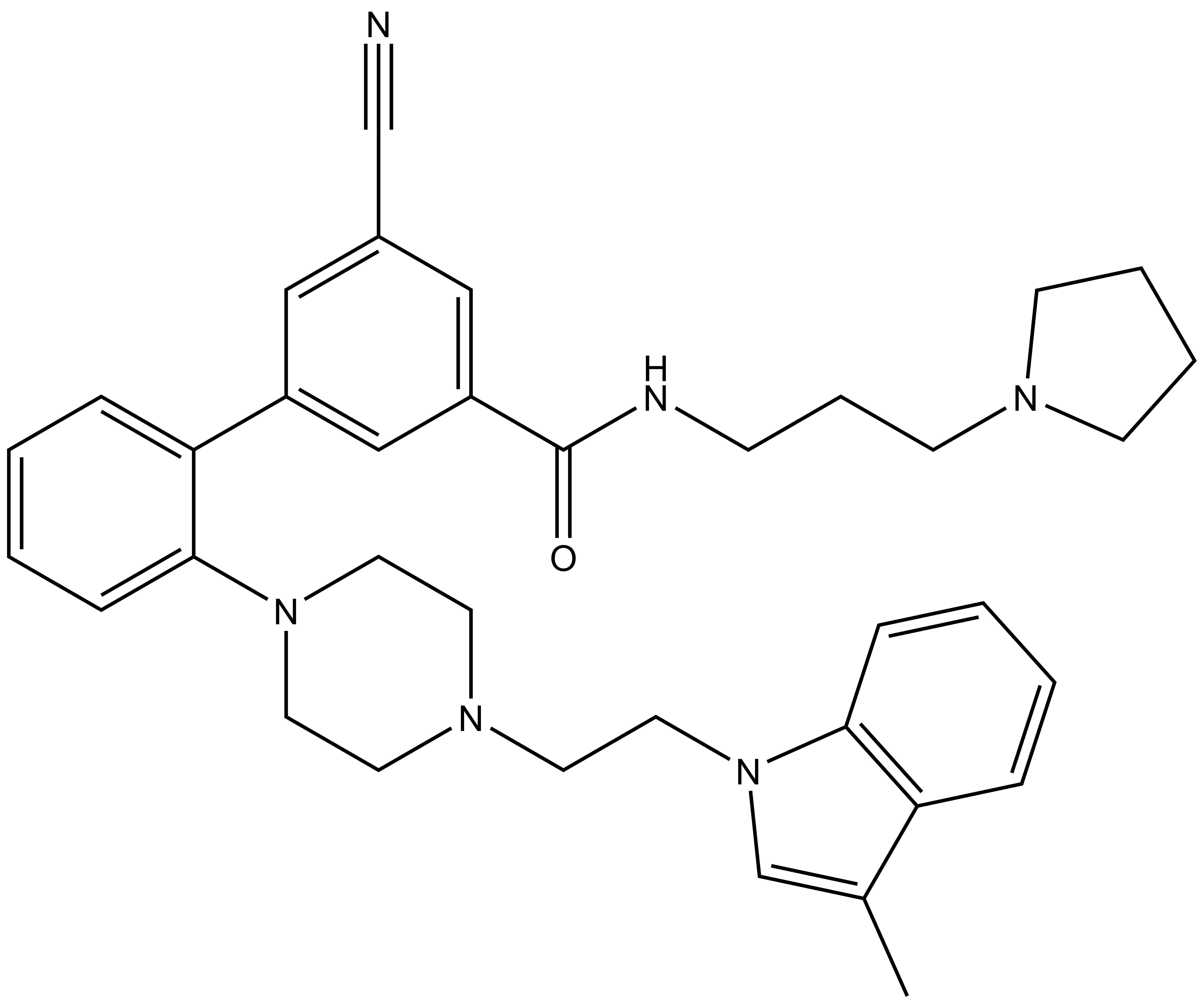
LLY-507 (IC50 < 15 nM) |
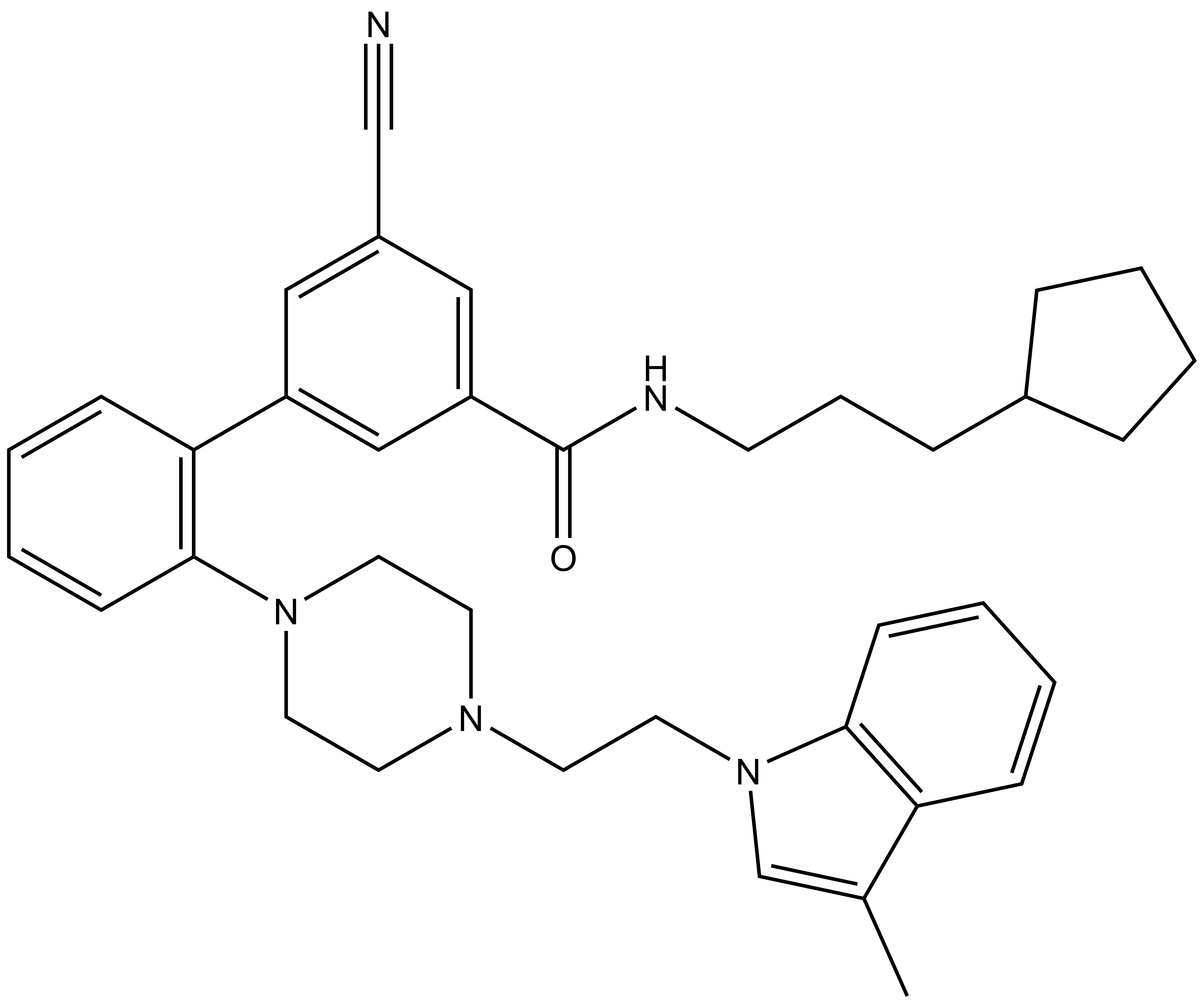
| SGC705 (IC50> 10,000 nM) |
A collaboration between the SGC and Eli Lilly and Company has resulted in the discovery of LLY-507, a chemical probe for SMYD2 (a protein lysine methyltransferase). LLY-507 is a potent inhibitor of SMYD2 with in vitro IC50 <15 nM and >100-fold selectivity over other methyltransferases and other non-epigenetic targets. LLY-507 has been shown to inhibit p53K370 monomethylation in cells with an IC50 ~600 nM.
Note: After LLY-507 was released, off-target screening (in addition to those presented in the publication, doi:10.1074/jbc.M114.626861) was done. Here is a copy of the report from a functional screen of LLY-507 and its control SGC705 in the NIMH Psychoactive Drug Screening Program (PDSP). This report clearly shows more than 50% inhibition of a number of targets.
| Probe | Negative control | |
 |
|  |
LLY-507 (IC50 < 15 nM) |
| SGC705 (IC50> 10,000 nM) |
| Physical and chemical properties for LLY-507 | |
| Molecular weight | 574.3 |
| Molecular formula | C36H42N6O |
| IUPAC name | 5-(2-(4-(2-(9-methyl-7-aza-bicyclo[4.3.0]nona-1(6),2,4,8-tetraen-7-yl)-ethyl)-piperazin-1-yl)-phenyl)-3-((3-(pyrrolidin-1-yl)-propylamino)-formyl)-benzonitrile |
| MollogP | 5.891 |
| PSA | 54.28 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 12 |
| No. of hydrogen bond acceptors | 5 |
| No. of hydrogen bond donors | 1 |
| Physical and chemical properties for SGC705 (Negative Control) | |
| Molecular weight | 573.3 |
| Molecular formula | C37H43N5O |
| IUPAC name | 3-((3-cyclopentyl-propylamino)-formyl)-5-(2-(4-(2-(9-methyl-7-aza-bicyclo[4.3.0]nona-1(6),2,4,8-tetraen-7-yl)-ethyl)-piperazin-1-yl)-phenyl)-benzonitrile |
| MollogP | 7.504 |
| PSA | 50.49 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 12 |
| No. of hydrogen bond acceptors | 4 |
| No. of hydrogen bond donors | 1 |
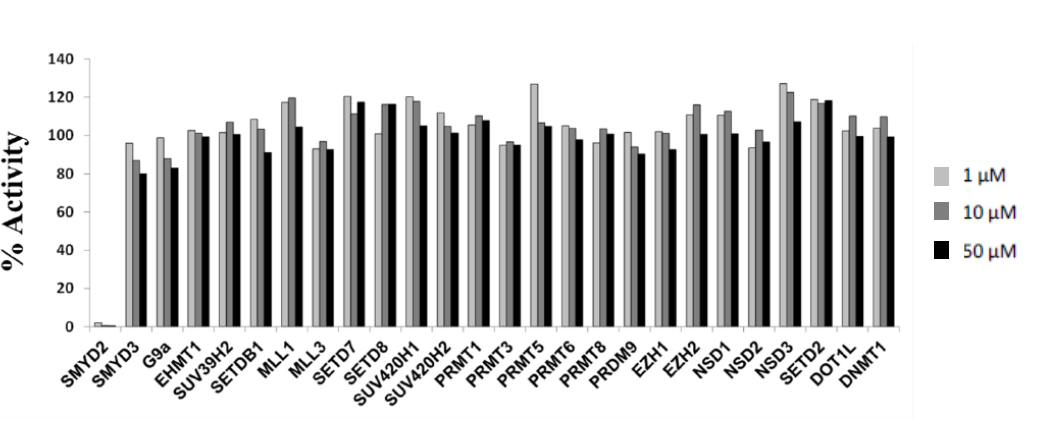
Effect of LLY-507 on the activity of 27 protein methyltransferases as well as DNMT1.
Mechanism of Action
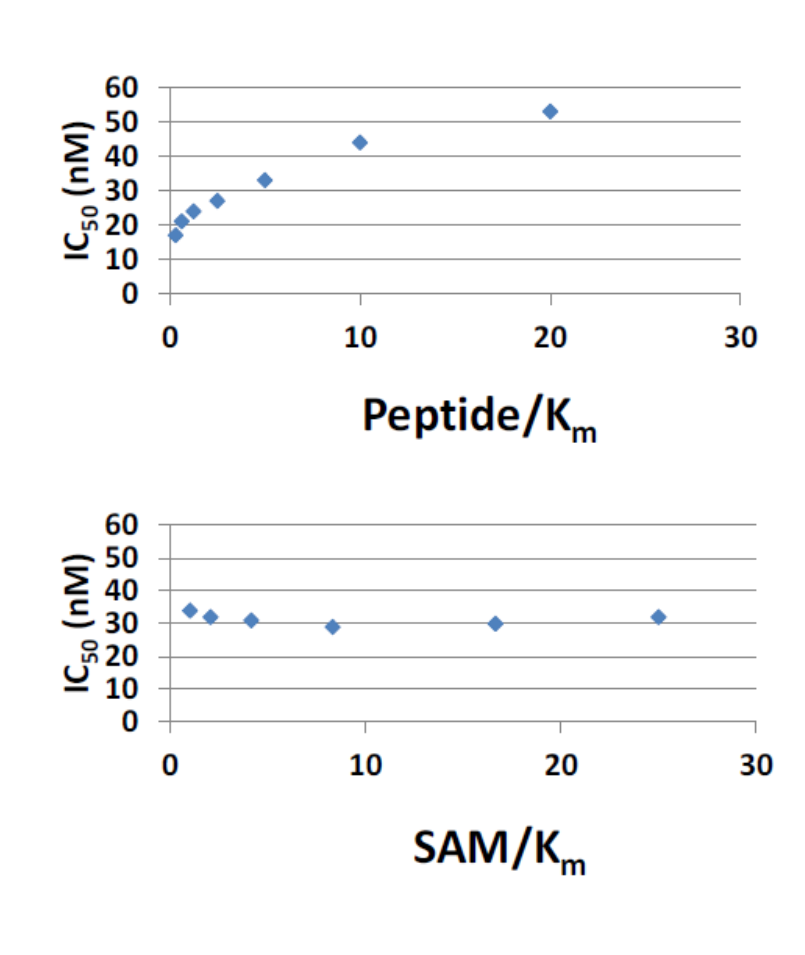
Cellular Activity
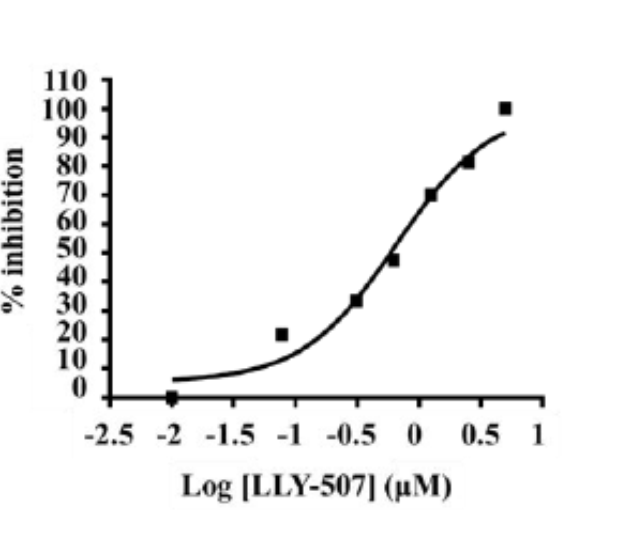
Dose dependent inhibition of p53 K370 me1 by LLY-507 in KYSE-150 cells stably expressing SMYD2 as measured by a Meso Scale Discovery sandwich ELISA assay (IC50 = 0.6 microM).
LLY-507, a Cell-Active, Potent and Selective Inhibitor of Protein Lysine Methyltransferase SMYD2 J. Biol. Chem. doi:10.1074/jbc.M114.626861
Hannah Nguyen, Abdellah Allali-Hassani, Stephen Antonysamy, Shawn Chang, Lisa Hong Chen, Carmen Curtis, Spencer Emtage, Li Fan, Tarun Gheyi, Fengling Li, Shichong Liu, Joseph R. Martin, David Mendel, Jonathan B. Olsen, Laura Pelletier, Tatiana Shatseva, Song Wu, Feiyu Fred Zhang, Cheryl H. Arrowsmith, Peter J. Brown, Robert M. Campbell, Benjamin A. Garcia, Dalia Barsyte-Lovejoy, Mary Mader and Masoud Vedadi.
PDB: 4WUY
Main features