Click here to obtain this probe.
| Probe | Negative control | |
 |
|  |
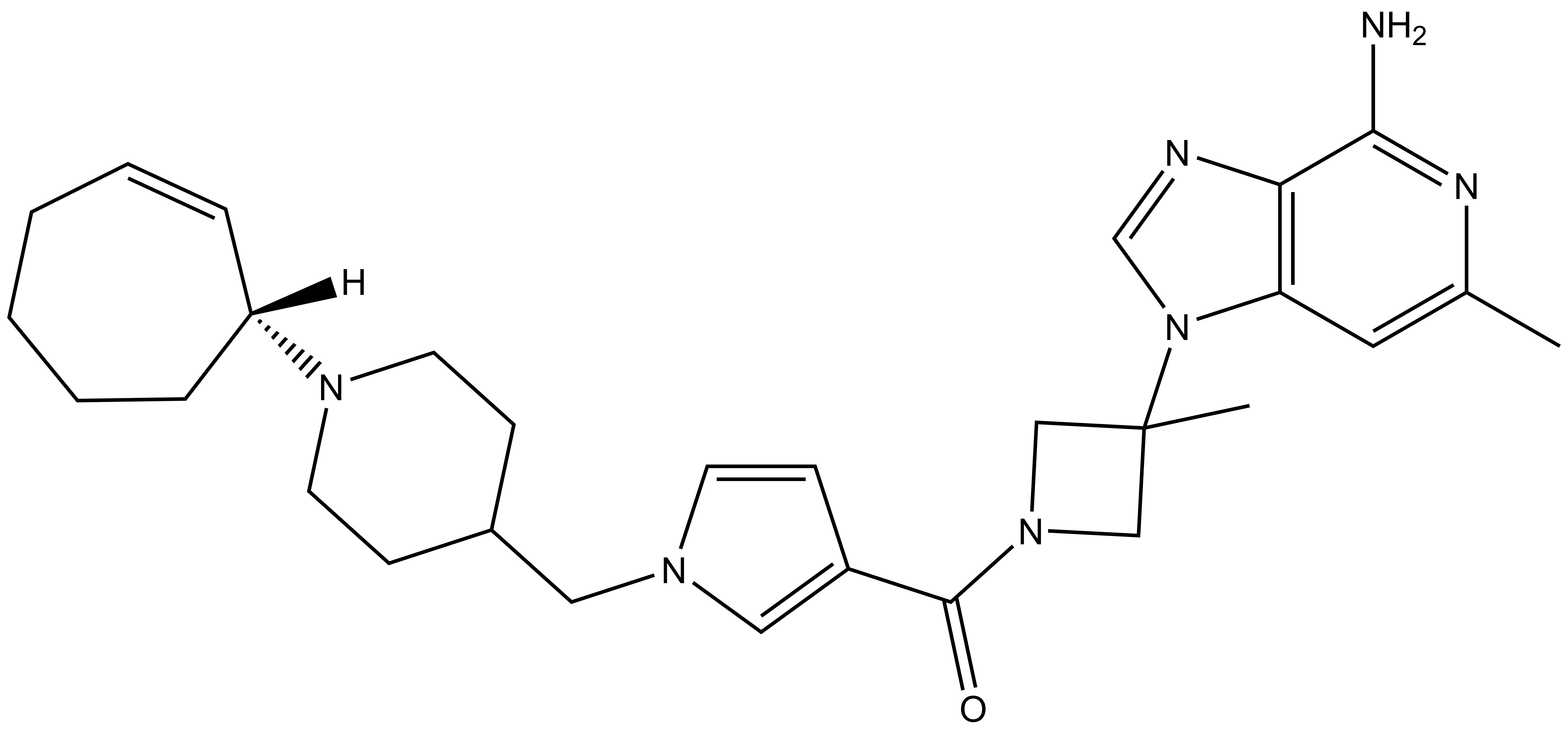
PFI-5 |
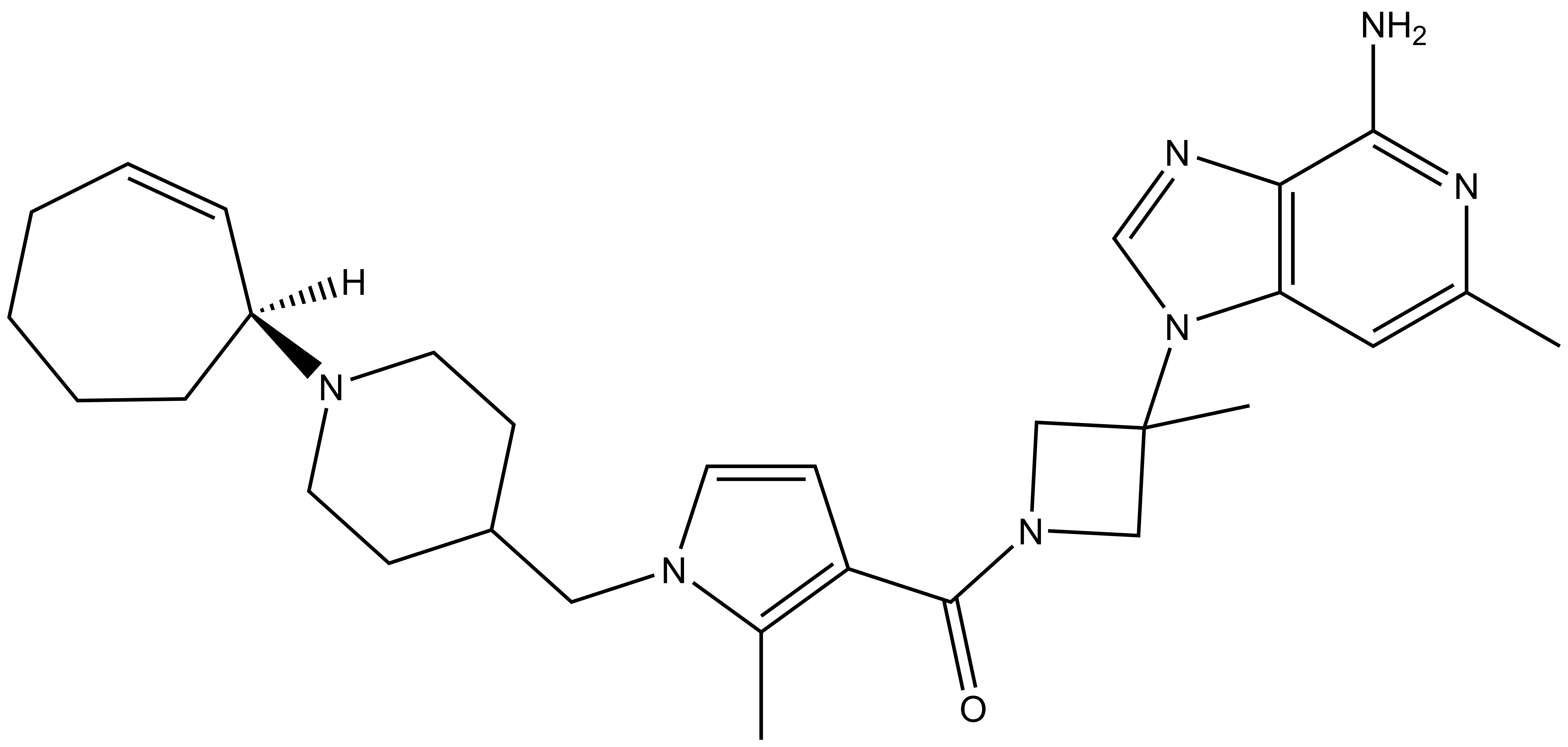
| PFI-5N |
A collaboration between Pfizer and the SGC has resulted in the discovery of PFI-5, a potent inhibitor of SMYD2 with mixed MOA. PFI-5 has a unique chemotype relative to the current SMYD2 chemical probes LLY-507 and BAY-598. PFI-5 inhibits in vitro methylation of p53K370 with IC50 = 9 nM and has more than 100-fold selectivity over other histone methyltransferases and other non-epigenetic targets. PFI-5 inhibits the methylation of p53K370 in cells with IC50 = 0.9 µM. A control compound, PFI-5N, has also been developed which inhibits the in vitro methylation of p53K370 with IC50 > 10 micromolar.
| Probe | Negative control | |
 |
|  |
PFI-5 |
| PFI-5N |
| Physical and chemical properties for PFI-5 | |
| Molecular weight | 561.3 |
| Molecular formula | C29H39N7O.C2H4O2 |
| IUPAC name | (3-(2-amino-4-methyl-3,7,9-triaza-bicyclo[4.3.0]nona-1(6),2,4,8-tetraen-7-yl)-3-methyl-azetidin-1-yl)-(1-((1-(cyclohept-2-enyl)-piperidin-4-yl)-methyl)-1H-pyrrol-3-yl)-methanone;acetic acid |
| MollogP | 2.93 |
| PSA | 93.76 |
| No. of chiral centres | 1 |
| No. of rotatable bonds | 6 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 3 |
| Physical and chemical properties for PFI-5N (Negative Control) | |
| Molecular weight | 575.4 |
| Molecular formula | C30H41N7O.C2H4O2 |
| IUPAC name | (3-(2-amino-4-methyl-3,7,9-triaza-bicyclo[4.3.0]nona-1(6),2,4,8-tetraen-7-yl)-3-methyl-azetidin-1-yl)-(1-((1-(cyclohept-2-enyl)-piperidin-4-yl)-methyl)-2-methyl-1H-pyrrol-3-yl)-methanone;acetic acid |
| MollogP | 3.191 |
| PSA | 63.57 |
| No. of chiral centres | 1 |
| No. of rotatable bonds | 6 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 3 |
Main features