This probe is available from Tocris, Sigma and Cayman Chemical
| Probe | Negative control | |
 |
|  |
SGC-CBP30 |
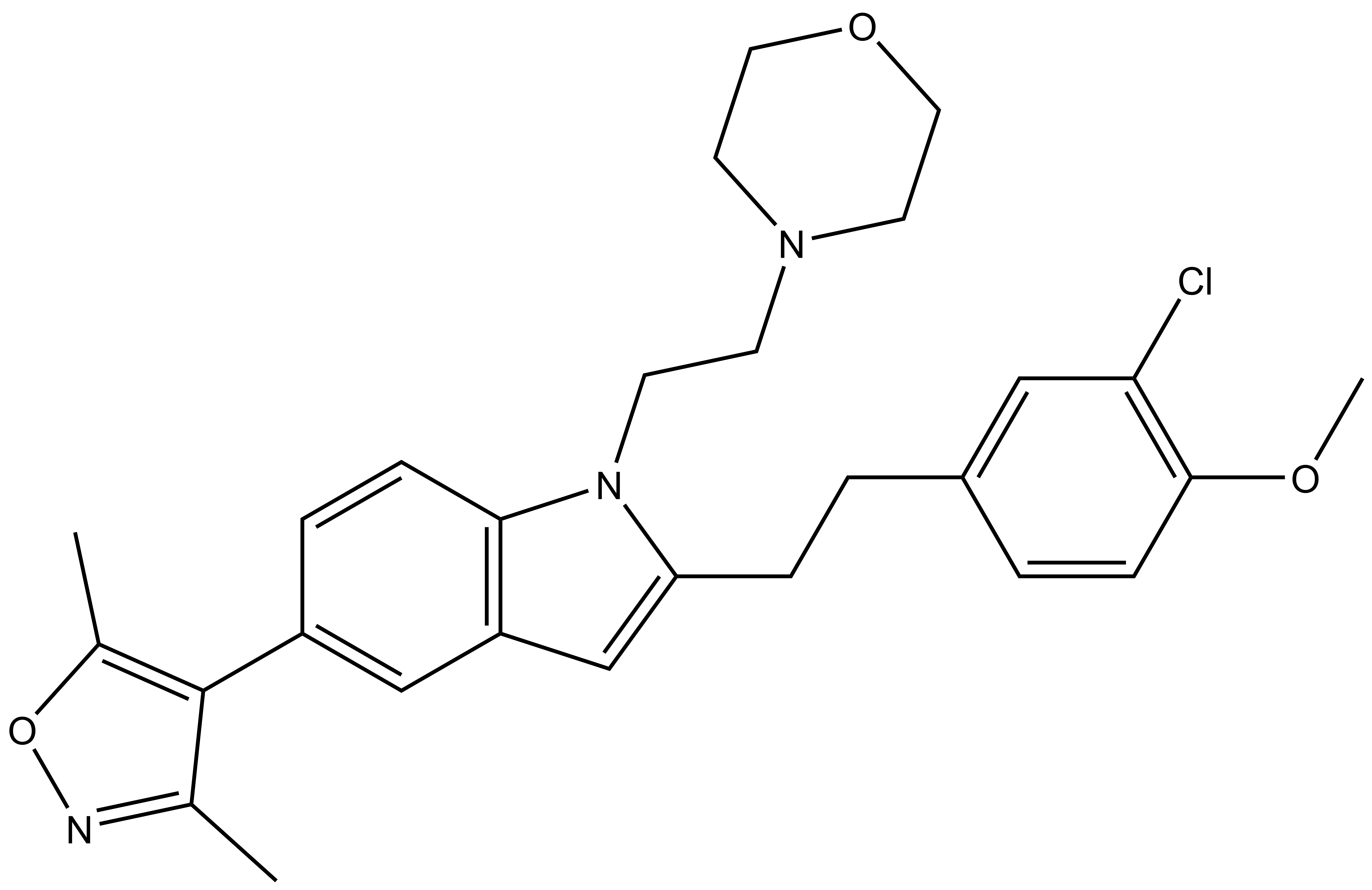
| BDOIA513 |
| In vitro Potency | |
|---|---|
| Assay | IC50/Kd µM |
| CREBBP (Alphascreen) | 0.069 |
| CREBBP (BLI) | 0.041 |
| CREBBP (ITC) | 0.021 |
| EP300 (ITC) | 0.038 |
CREBBP (CBP) and EP300 are general transcriptional co-activators, which are involved in many biological processes like maintenance of genomic stability by affecting DNA replication and DNA repair as well as cell growth, transformation and development. They also play and essential role in neuronal plasticity/ memory formation hematopoiesis and energy homeostasis as demonstrated in a variety of mouse models. They possess both acetyl-transferase enzymatic and bromodomain containing regions. Through acetylation of non-histone proteins CREBBP can have a positive or negative effect on transcriptional regulation by affecting protein- protein interactions, protein-DNA interactions, nuclear retention or protein half-life.
Mutations of CREBBP and EP300 lead to Rubinstein-Taybi syndrome (RTS), characterised by growth retardation, facial abnormalities, organ abnormalities, mental retardation and altered tumor susceptibility. Chromosomal translocations of CREBBP or EP300 with MOZ, MLL have been observed in acute myeloid leukemia.
CREBBP has also been associated with Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s disease, a neurodegenerative disease with progressive degeneration of motor neurons in the brain and spinal cord, Alzheimer's disease and poly glutamine repeat diseases such as Spinal and Bulbar Muscular Atrophy and Huntington’s disease.
We have developed an inhibitor, SGC-CBP30* against the CREBBP and EP300 Bromodomains.
* This is officially pronounced SGC-CBP-three-oh
SGC-CBP30N (BDOIA513) is the negative control for SGC-CBP30. This compound has moderate activity for CREBBP (Thermal shift 2°C compared to 9.7°C for SGC-CBP30). This compound is inactive against BRD4 (Thermal shift 0.4°C compared to 1.8°C for SGC-CBP30)
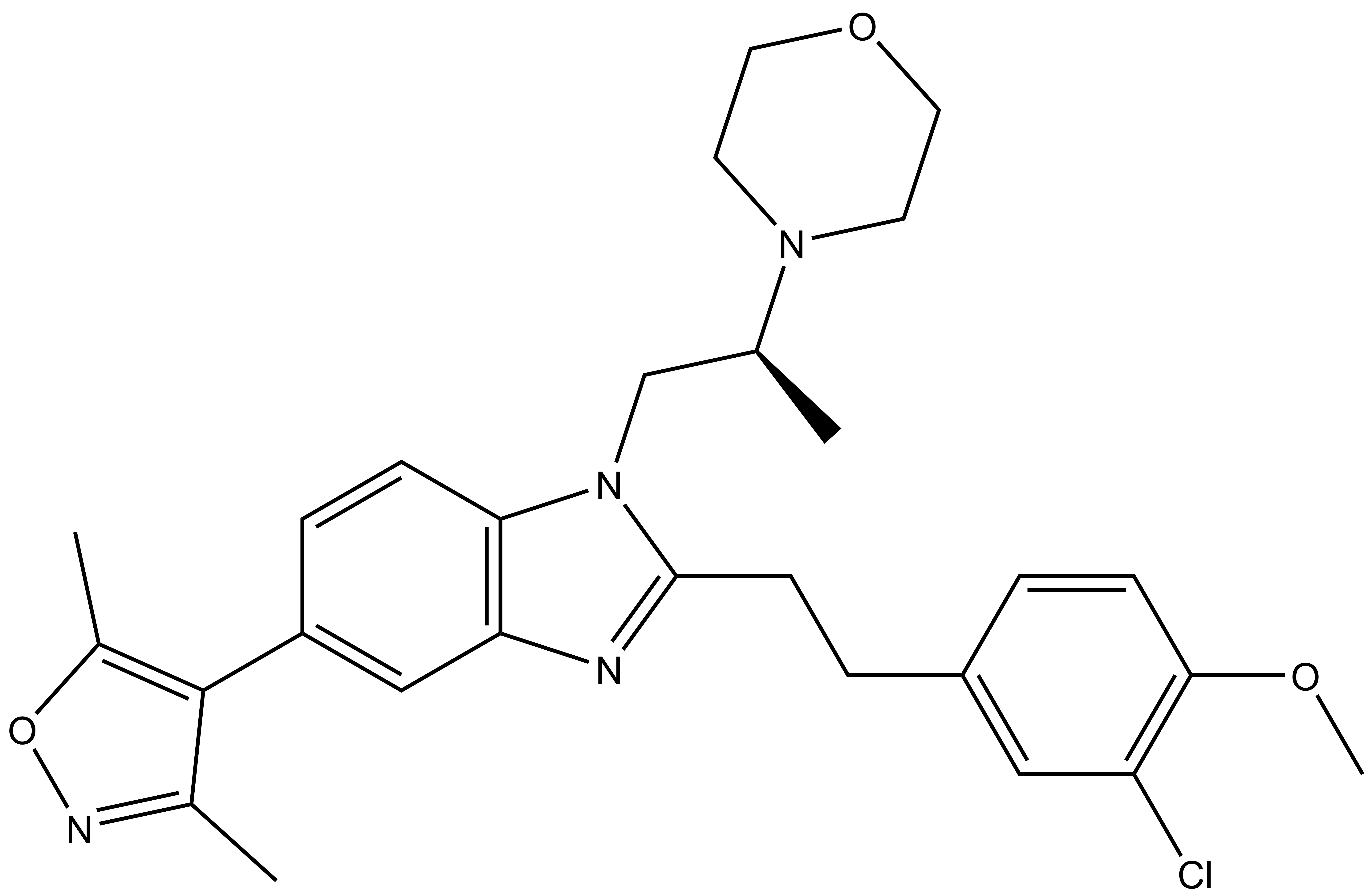
| SGC-CBP30 |
 |
| 8-(3-chloro-4-methoxy-phenethyl)-4-(3,5-dimethyl-isoxazol-4-yl)-9-(2-(morpholin-4-yl)-propyl)-7,9-diaza-bicyclo[4.3.0]nona-1(6),2,4,7-tetraene Click here to download SDF file |
| Physical and chemical properties | |
|---|---|
| Molecular weight | 508.2 |
| Molecular formula | C28H33ClN4O3 |
| IUPAC name | 8-(3-chloro-4-methoxy-phenethyl)-4-(3,5-dimethyl-isoxazol-4-yl)-9-(2-(morpholin-4-yl)-propyl)-7,9-diaza-bicyclo[4.3.0]nona-1(6),2,4,7-tetraene |
| logP | 4.89 |
| PSA | 51.8 |
| Storage | 2-8°C as powder. NB making aliquots rather than freeze-thawing is recommended |
| Dissolution | Soluble in DMSO at least up to 50mM |
Measured Properties
Differential Scanning Fluorimetry (DSF)
Thermal melting experiments were carried out using an Mx3005p Real Time PCR machine (Stratagene). Proteins were buffered in 10 mM HEPES pH 7.5, 500 mM NaCl and assayed in a 96-well plate at a final concentration of 2 µM in 20 µl volume. BDOIA518 was added at a final concentration of 10 µM. SYPRO Orange (Molecular Probes) was added as a fluorescence probe at a dilution of 1:1000. Excitation and emission filters for the SYPRO-Orange dye were set to 465 nm and 590 nm, respectively. The temperature was raised with a step of 3 °C per minute from 25 °C to 96 °C and fluorescence readings were taken at each interval. The temperature dependence of the fluorescence during the protein denaturation process was fit to the Boltzmann equation.
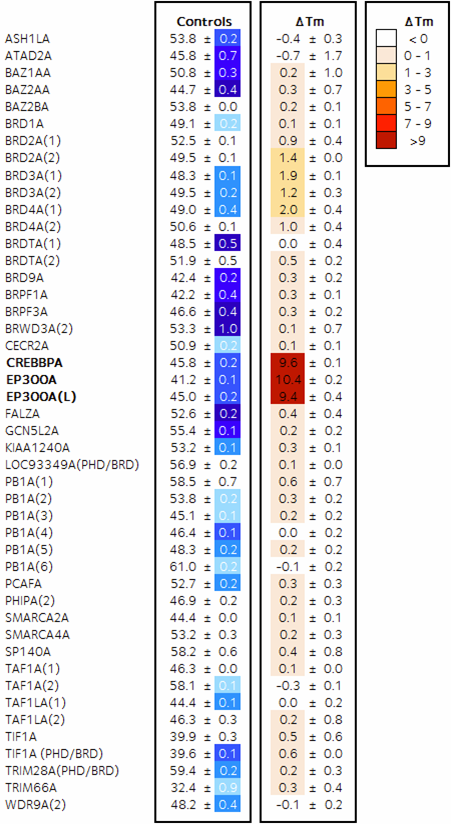
Activity against BRD4(1) by ITC 0.86 mM: 40 fold selective for CBP. (click for larger version)
SGC-CBP30 was profiled against the CEREP panel of Receptors, Ion Channels and Enzymes with the following results
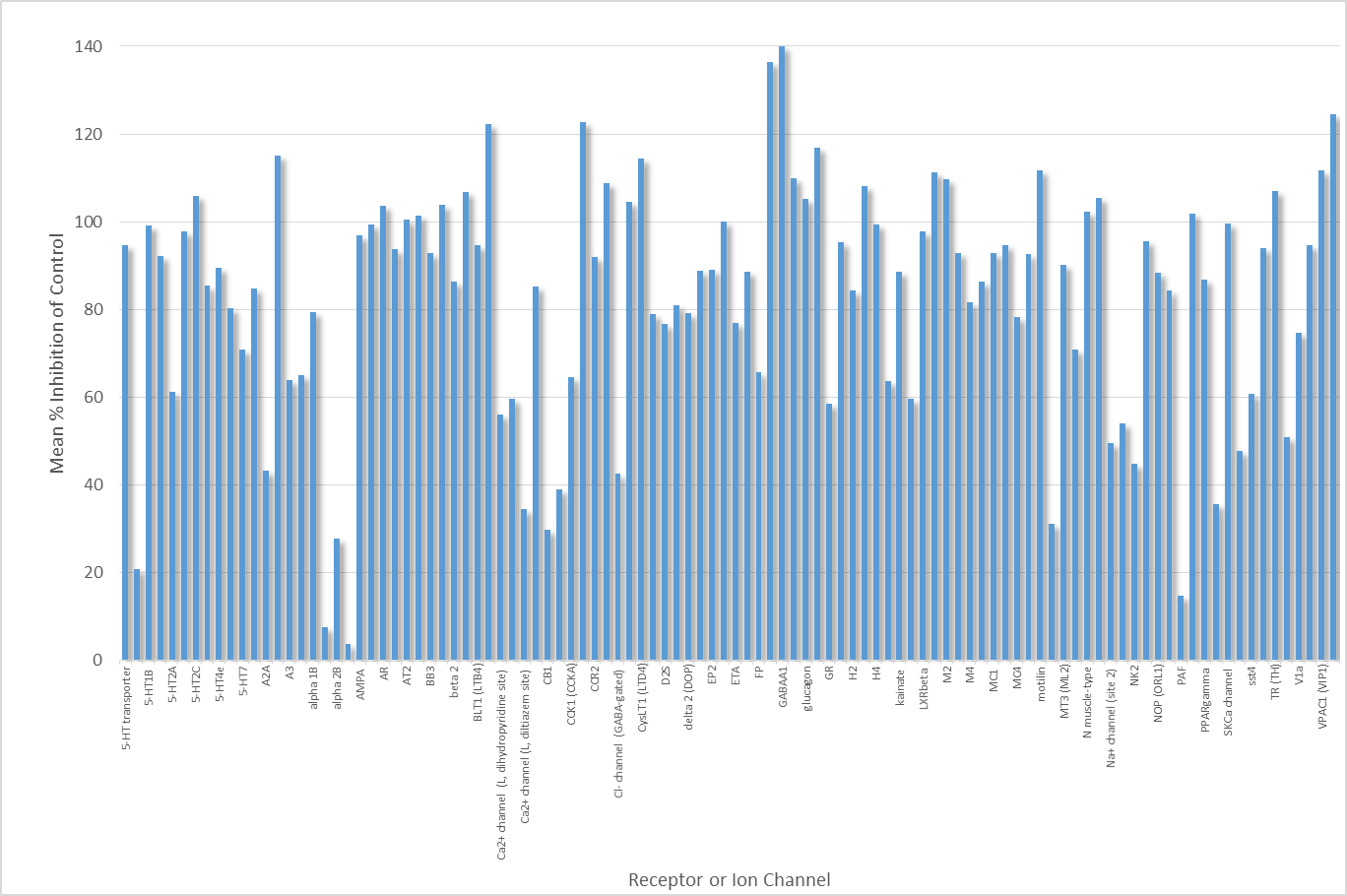
(click for larger version)
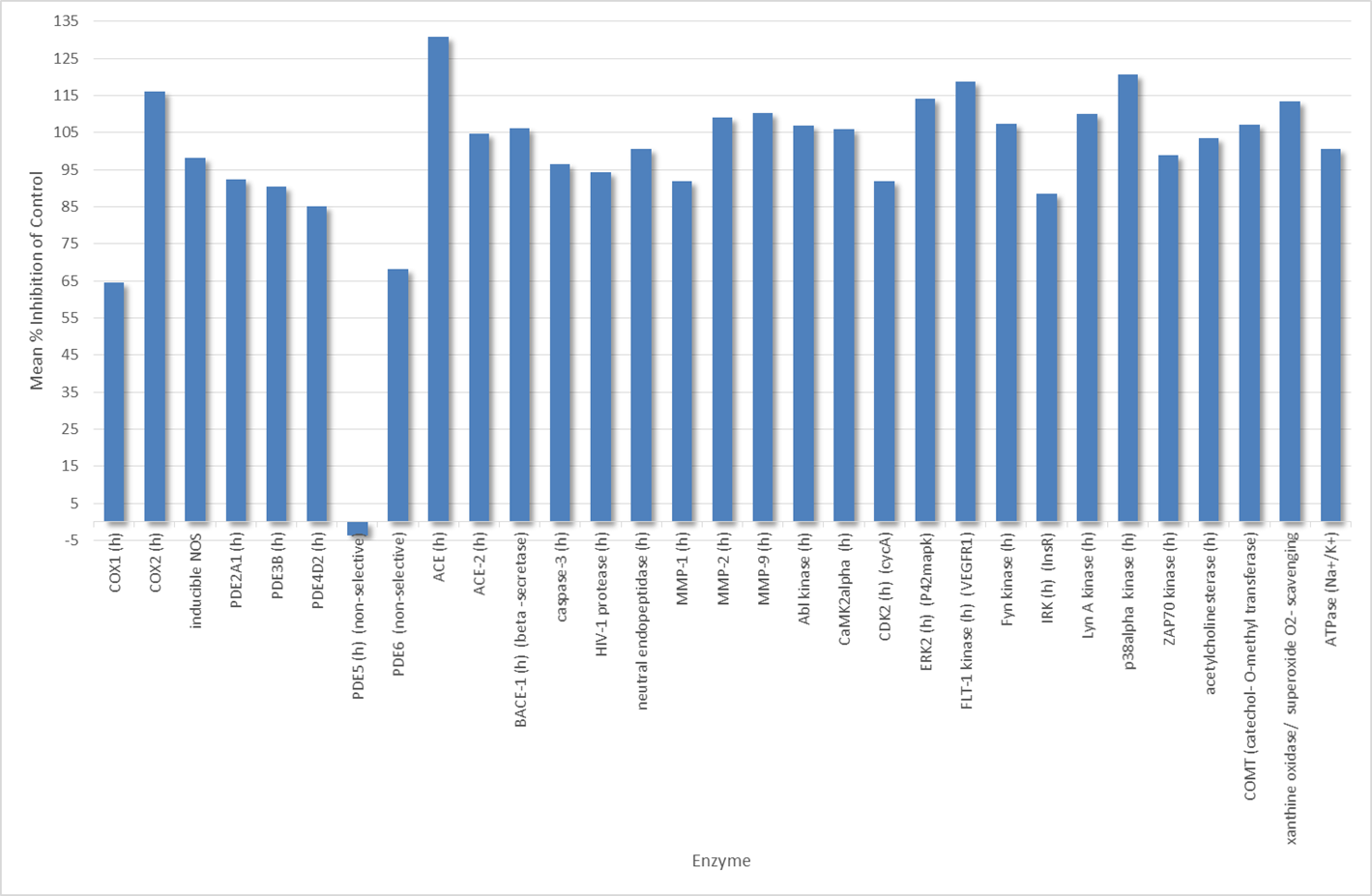
(click for larger version)
| Activity In cells | |
| CREBBP (3x bromodomain) (FRAP assay) | Accelerated FRAP recovery at 1 µM |
Moderate cytotoxicity in U2OS cells and HeLa cells.
Discovery and Optimization of Small-Molecule Ligands for the CBP/p300 Bromodomains
Duncan A. Hay, Oleg Fedorov, Sarah Martin, Dean C. Singleton, Cynthia Tallant,Christopher Wells, Sarah Picaud, Martin Philpott, Octovia P. Monteiro, Catherine M. Rogers, Stuart J. Conway, Timothy P. C. Rooney, Anthony Tumber, Clarence Yapp,Panagis Filippakopoulos, Mark E. Bunnage,Susanne Müller, Stefan Knapp, Christopher J. Schofield, and Paul E. Brennan
J. Am. Chem. Soc., 2014, doi: 10.1021/
CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses.
Hammitzsch A, Tallant C, Fedorov O, O'Mahony A, Brennan PE, Hay DA, Martinez FO, Al-Mossawi MH, de Wit J, Vecellio M, Wells C, Wordsworth P, Müller S, Knapp S, Bowness P.
Proc Natl Acad Sci U S A. 2015, 112(34), 10768-73. doi: 10.1073/pnas.1501956112
SGC-CBP30 has been co-crystallised with CREBBP and is deposited in the PDB - 4NR7