| Probe | Negative control | |
 |
|  |
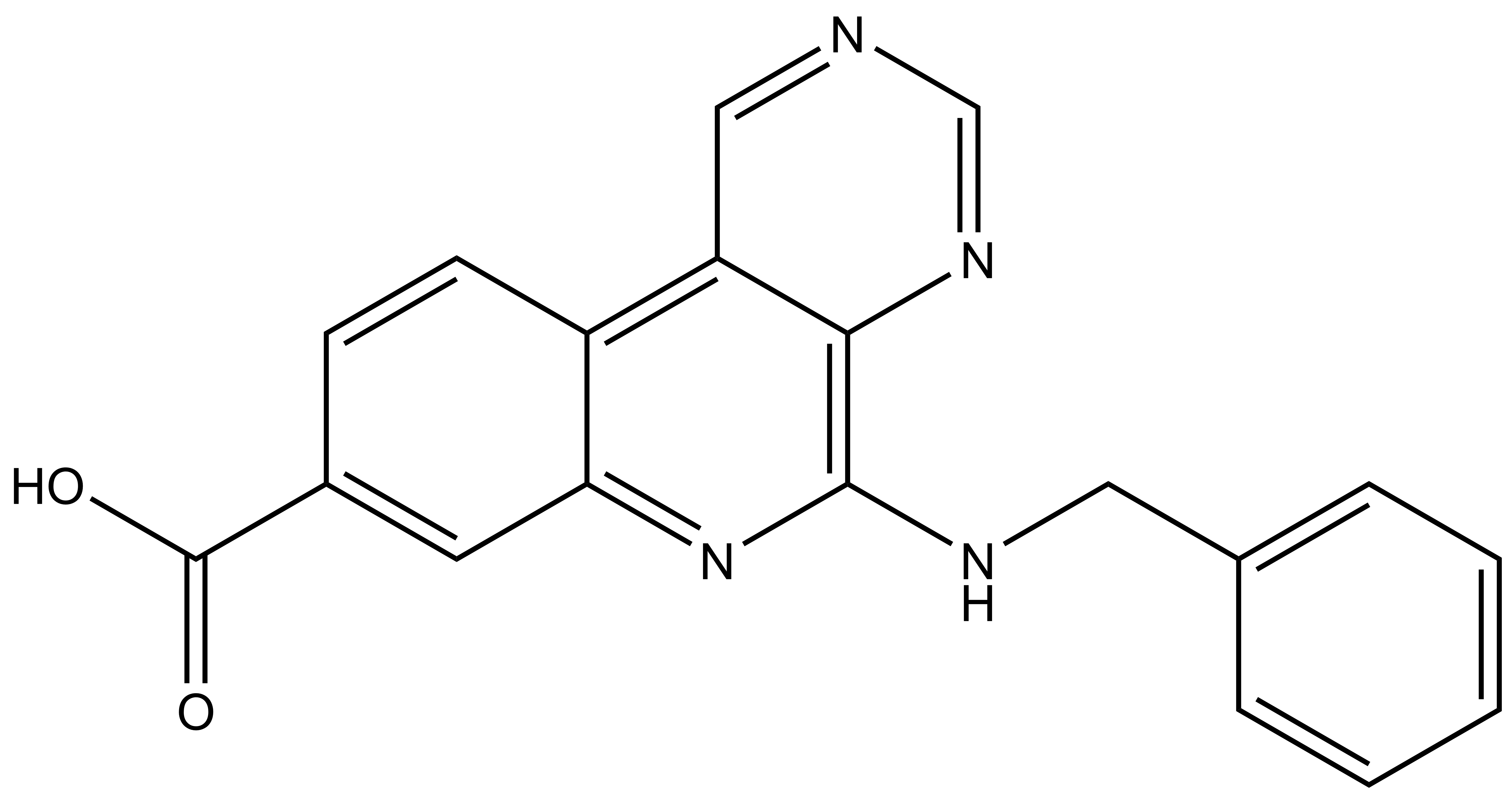
SGC-CK2-2 |
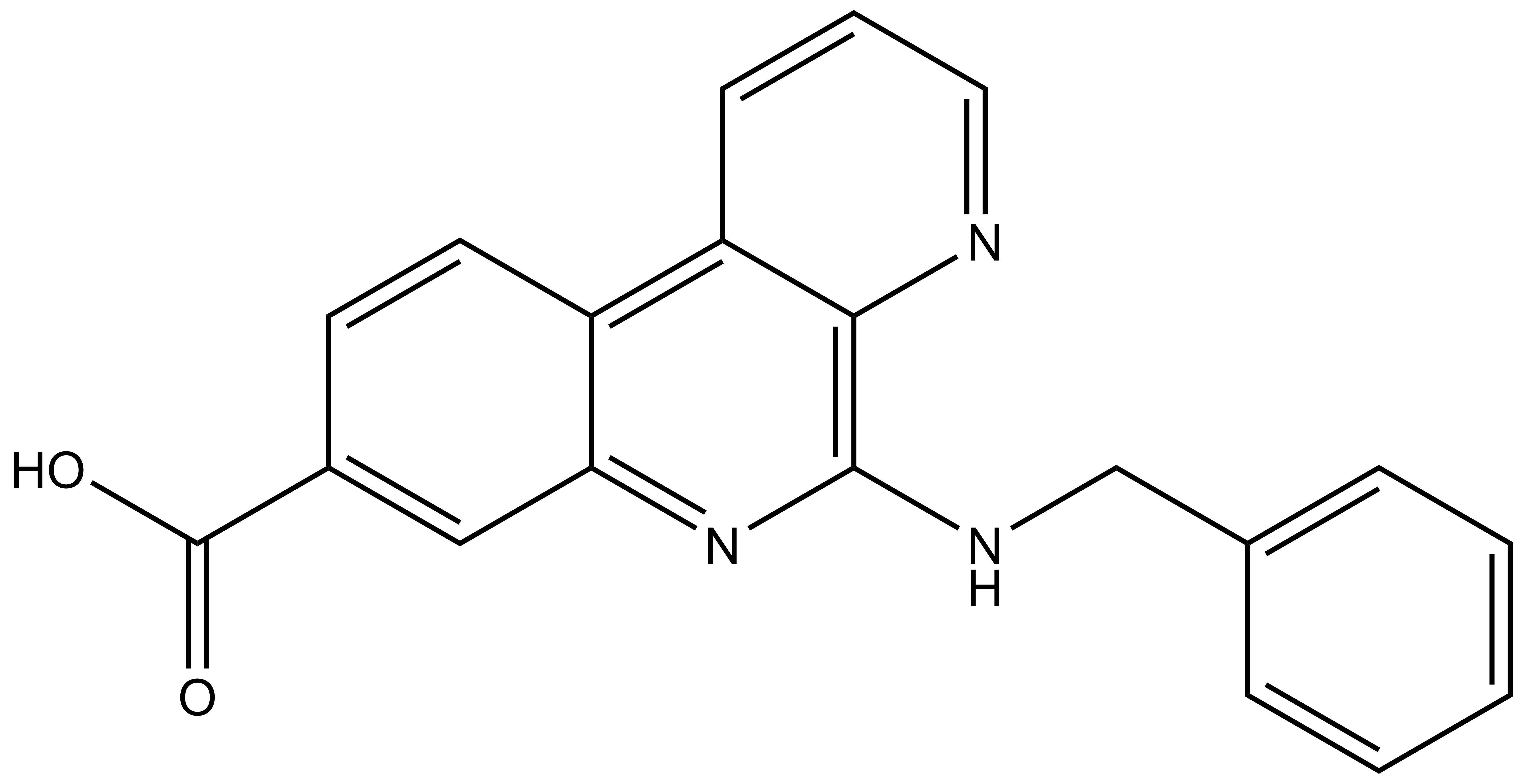
| SGK-CK2-2N |
From a library of naphthyridines, we identified a potent and cell-active chemical probe (SGC-CK2-2) that inhibits casein kinase 2 (CK2/CSNK2). Comprehensive evaluation of kinome-wide selectivity confirmed that this CK2 probe demonstrates excellent selectivity. A structurally similar naphthyridine (SGC-CK2-2N) was characterized as a negative control that does not inhibit CK2 and exhibits exceptional selectivity when profiled broadly. Our CK2 chemical probe is not broadly antiproliferative when tested against 16 cancer cell lines of diverse origins. Versus a published CK2 chemical probe (SGC-CK2-1), our scaffold is a distinct chemotype, has non-overlapping kinase off-targets, and demonstrates improved kinetic solubility. Our chemical probe set can be used by the community to further characterize the diverse roles of CK2.
Biological activity summary:
| Probe | Negative control | |
 |
|  |
SGC-CK2-2 |
| SGK-CK2-2N |
| Physical and chemical properties for SGC-CK2-2 | |
| Molecular weight | 330.35 |
| Molecular formula | C19H14N4O2 |
| IUPAC name | 5-(benzylamino)pyrimido[4,5-c]quinoline-8-carboxylic acid |
| MollogP | 3.11 |
| PSA | 86.41 |
| No. of chiral centers | 0 |
| No. of rotatable bonds | 4 |
| No. of hydrogen bond acceptors | 5 |
| No. of hydrogen bond donors | 2 |
| Storage | Stable as a solid at room temperature. DMSO stock solutions (up to 100 mM) are stable at -20oC |
| Dissolution | Soluble in DMSO up to 100 mM |
| Physical and chemical properties for SGC-CK2-2N | |
| Molecular weight | 329.36 |
| Molecular formula | C20H15N3O2 |
| IUPAC name | 5-(benzylamino)benzo[f][1,7]naphthyridine-8-carboxylic acid |
| MollogP | 3.7 |
| PSA | 74.05 |
| No. of chiral centers | 0 |
| No. of rotatable bonds | 4 |
| No. of hydrogen bond acceptors | 4 |
| No. of hydrogen bond donors | 2 |
| Storage | Stable as a solid at room temperature. DMSO stock solutions (up to 10 mM) are stable at -20oC |
| Dissolution | Soluble in DMSO up to 10 mM |
SMILES:
SGC-CK2-2: OC(C1=CC2=NC(NCC3=CC=CC=C3)=C4N=CN=CC4=C2C=C1)=O
SGC-CK2-2N: OC(C1=CC2=NC(NCC3=CC=CC=C3)=C4N=CC=CC4=C2C=C1)=O
InChI:
SGC-CK2-2: InChI=1S/C19H14N4O2/c24-19(25)13-6-7-14-15-10-20-11-22-17(15)18(23-16(14)8-13)21-9-12-4-2-1-3-5-12/h1-8,10-11H,9H2,(H,21,23)(H,24,25)
SGC-CK2-2N: InChI=1S/C20H15N3O2/c24-20(25)14-8-9-15-16-7-4-10-21-18(16)19(23-17(15)11-14)22-12-13-5-2-1-3-6-13/h1-11H,12H2,(H,22,23)(H,24,25)
InChIKey:
SGC-CK2-2: HEVVNKYNJCSHFA-UHFFFAOYSA-N
SGC-CK2-2N: VBKHXXPYOSWXSA-UHFFFAOYSA-N
Selectivity Profile
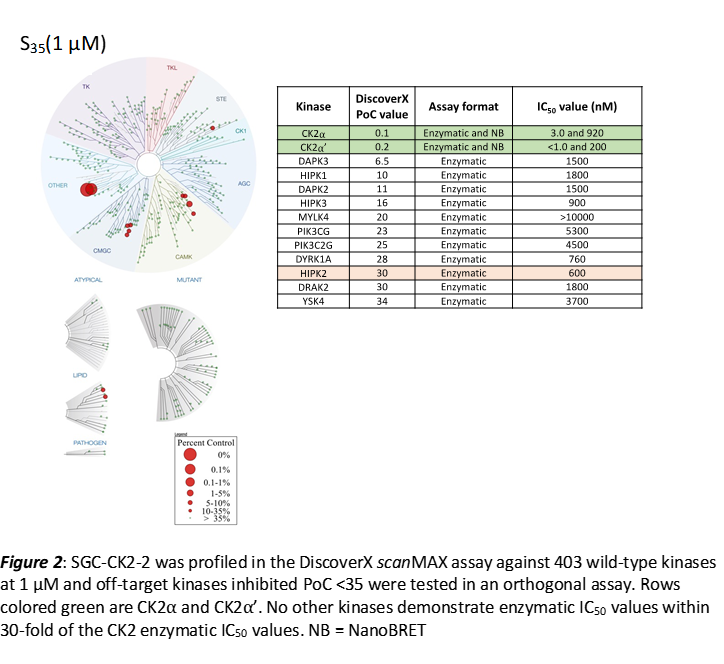
SGC-CK2-2 was profiled in the DiscoverX scanMAX assay against 403 wild-type kinases at 1 μM. Only 3 kinases showed PoC <10 giving an S10(1 μM) = 0.007. When the PoC <35 fraction was examined, 13 kinases were included (S35(1 μM) = 0.032). Potential off-targets within the S35(1 μM) fraction were tested via biochemical enzymatic assays plus NanoBRET target engagement assays for CK2⍺ and CK2⍺’. Data corresponding with off-target kinase activity is shown in the table below.
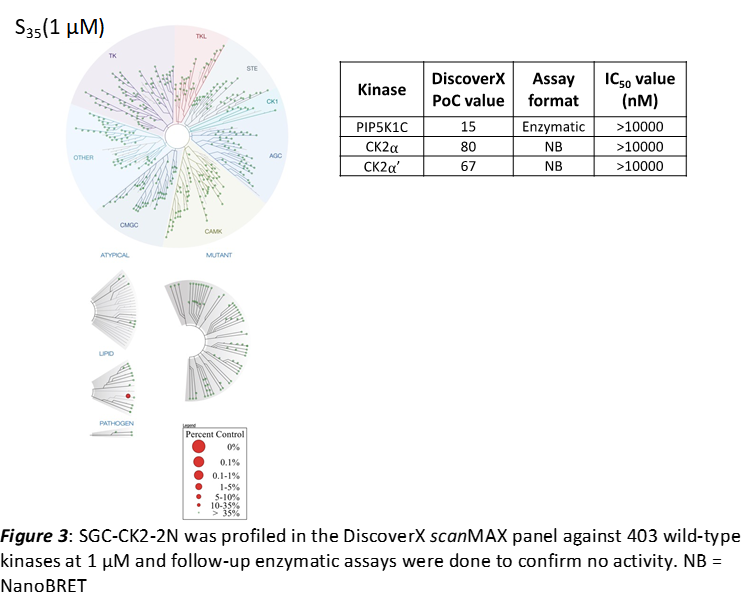
SGC-CK2-2N was also tested in the DiscoverX scanMAX panel and 1 kinase had a PoC <35 (S35(1 μM) = 0.002). The negative control was sent to Eurofins for testing in the enzyme assays for PIP5K1C, CK2⍺, and CK2⍺’. All results are in the table below.
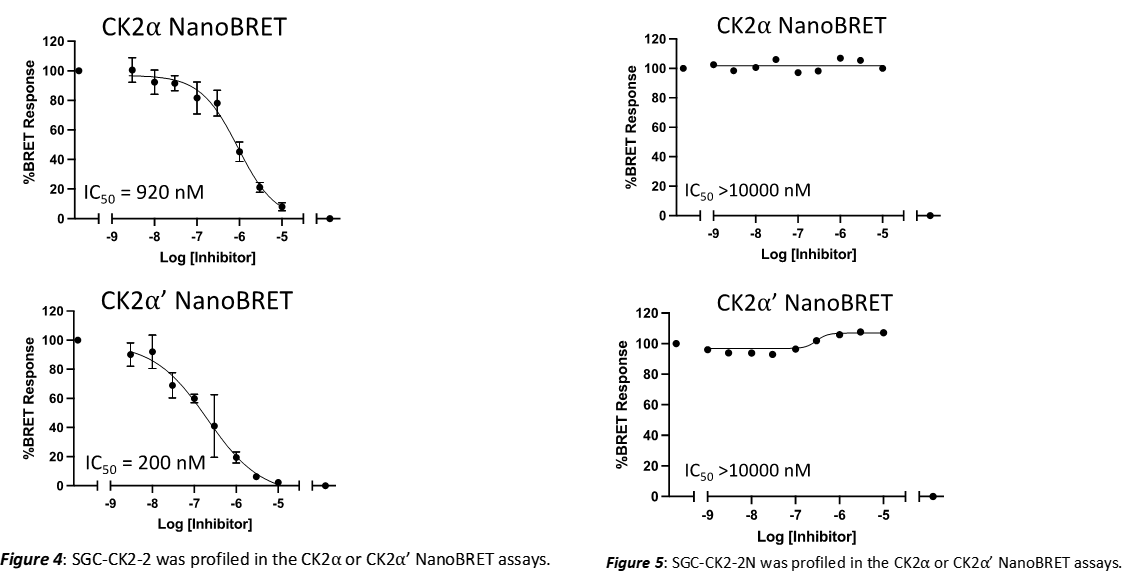
A NanoBRET assay was utilized to assess the binding affinity of SGC-CK2-2 to CK2⍺ and CK2⍺’. The negative control shows no binding affinity for CK2⍺ or CK2⍺’.
Davis-Gilbert, Z. W.; Krämer, A.; Dunford, J. E.; Howell, S.; Senbabaoglu, F.; Wells, C. I.; Bashore, F. M.; Havener, T. M.; Smith, J. L.; Hossain, M. A.; Oppermann, U.; Drewry, D. H.; Axtman, A. D. Discovery of a potent and selective naphthyridine-based chemical probe for casein kinase 2. ACS Med Chem Lett 2023, 14, 432–441; doi: 10.1021/acsmedchemlett.2c00530.
Davis-Gilbert, Z. W.; Krämer, A.; Dunford, J. E.; Howell, S.; Senbabaoglu, F.; Wells, C. I.; Havener, T. M.; Smith, J. L.; Hossain, M. A.; Oppermann, U.; Drewry, D. H.; Axtman, A. D. Discovery of a potent and selective naphthyridine-based chemical probe for casein kinase 2. ChemRxiv 2022, doi: 10.26434/chemrxiv-2022-05jcz.