SGC-UBD253
Chemical probe for the ubiquitin binding domain of HDAC6.
-
overview
-
selectivity profile
-
in vitro potency
-
cell based assay data
-
references
overview
| Probe | | Negative control |
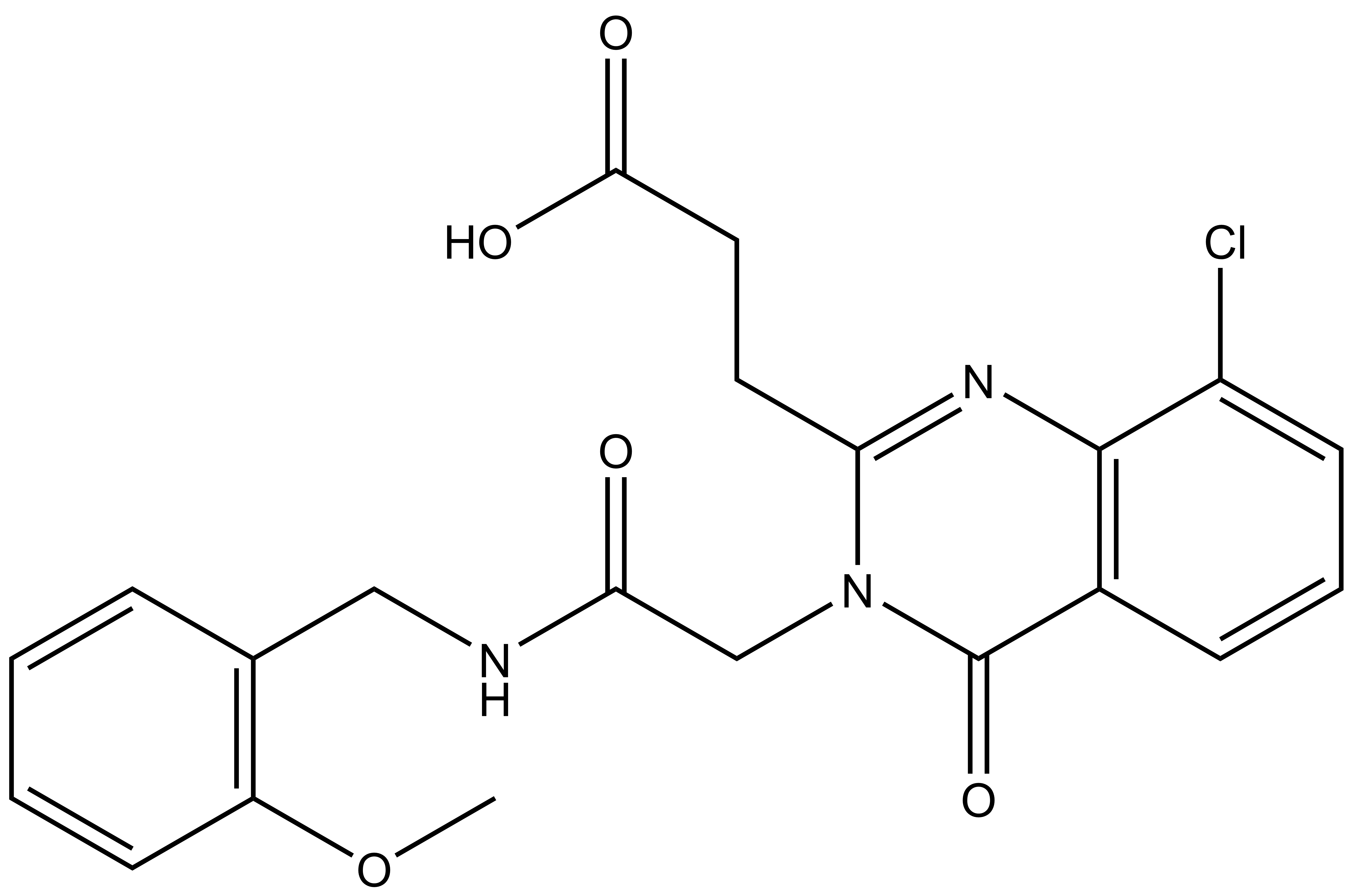 | | 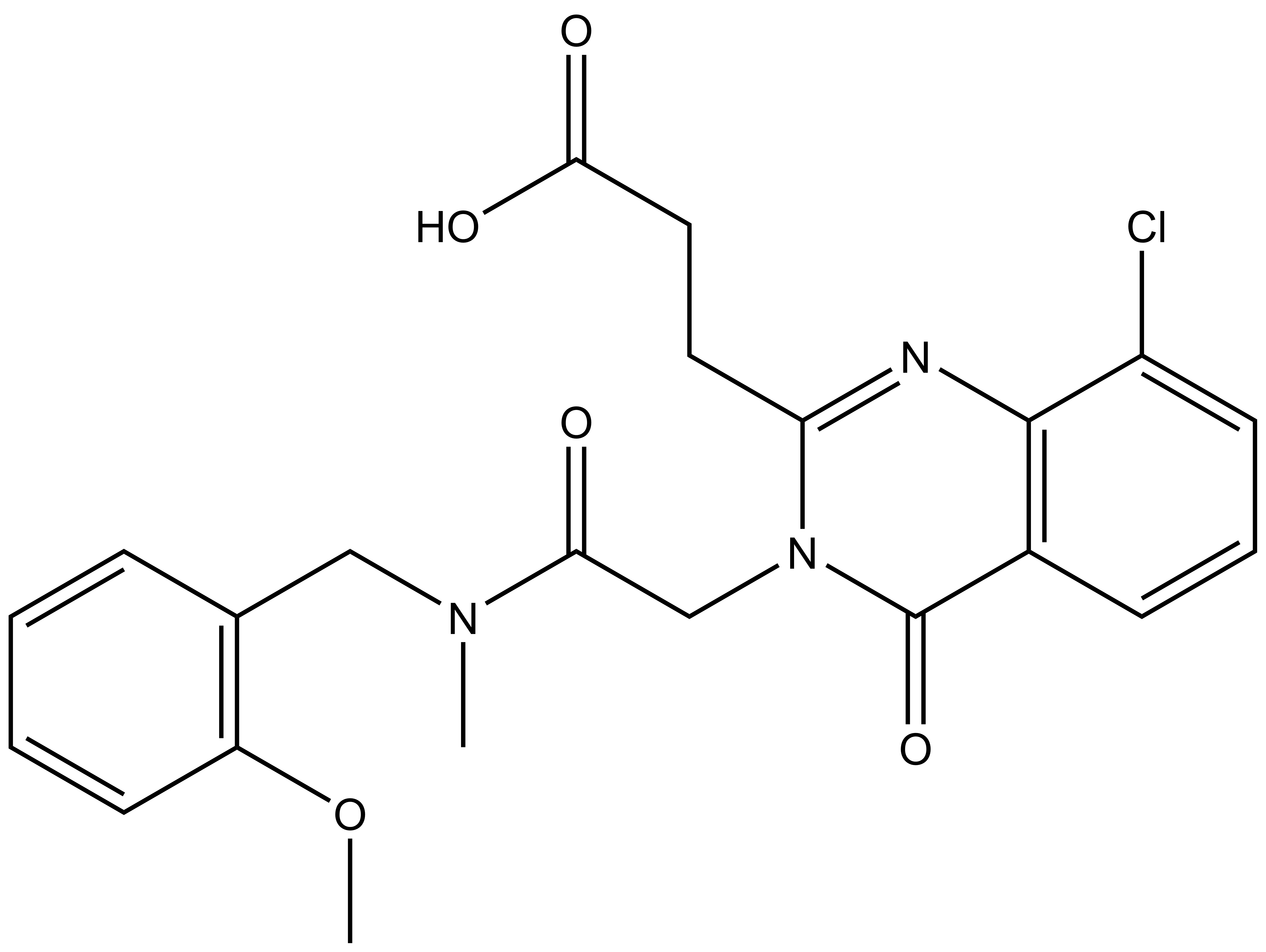 |
SGC-UBD253 | | SGC-UBD253N |
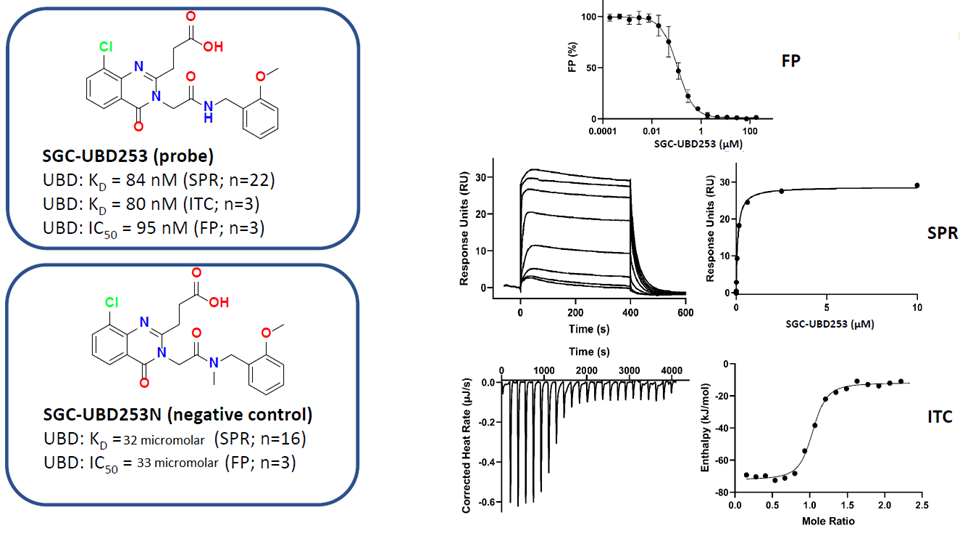
SGC in collaboration with Dr Mark Lautens’ at the University of Toronto’s Department of Chemistry has developed the first chemical probe SGC-UBD253 for the ubiquitin binding domain (UBD) of HDAC6. SGC-UBD253 binds potently to HDAC6 UBD with KD = 84 nM (SPR) and inhibits the HDAC6-ISG15 interaction with EC50 = 1.9 micromolar (nanoBRET). SGC-UBD253N is a closely related negative control with KD = 32 micromolar (SPR).
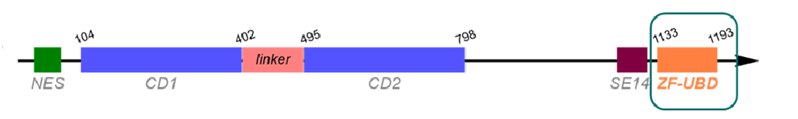
selectivity profile
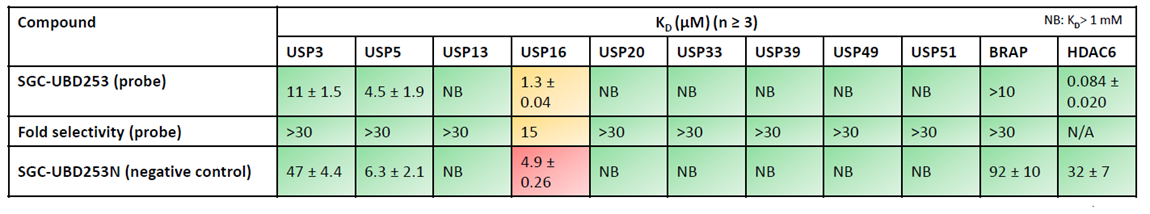
Using sequence alignment of Zf-UBD pocket, 10 UBD-containing proteins in addition to HDAC6 were purified and tested for direct binding by SPR.
in vitro potency
Three biophysical methods clearly show SGC-UBD253 potently (≤100 nM) binds HDAC6-UBD.
cell based assay data
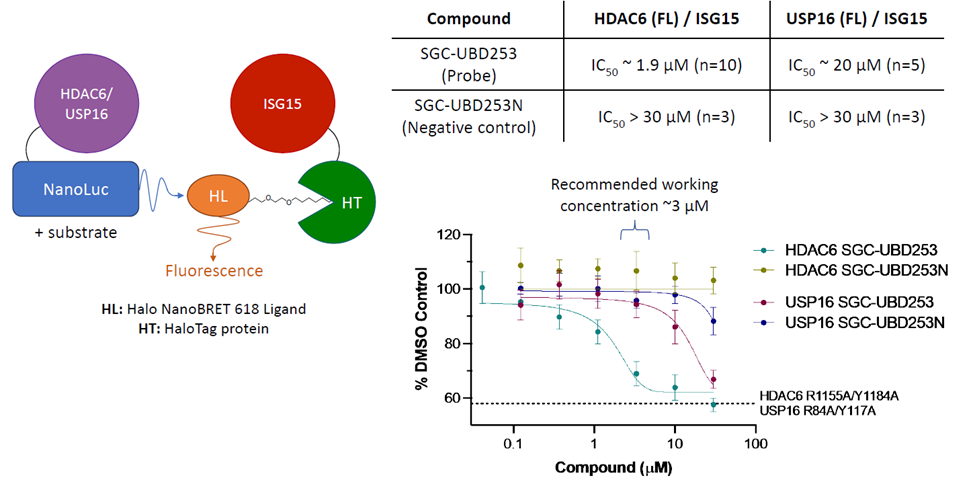
Using a nanoBRET assay, the chemical probe SGC-UBD253 considerably decreases the interaction between full-length HDAC6 with ISG15 relative to SGC-UBD253N.
references
- Ferreira de Freitas R, Harding RJ, Franzoni I, Ravichandran M, Mann MK, Ouyang H, Lautens M, Santhakumar V, Arrowsmith CH, Schapira M. Identification and Structure-Activity Relationship of HDAC6 Zinc-Finger Ubiquitin Binding Domain Inhibitors. J Med Chem. 2018 May 24;61(10):4517-4527. doi: 10.1021/acs.jmedchem.8b00258. PMID: 29741882
- Harding RJ, Ferreira de Freitas R, Collins P, Franzoni I, Ravichandran M, Ouyang H, Juarez-Ornelas KA, Lautens M, Schapira M, von Delft F, Santhakumar V, Arrowsmith CH. Small Molecule Antagonists of the Interaction between the Histone Deacetylase 6 Zinc-Finger Domain and Ubiquitin. J Med Chem. 2017 Nov 9;60(21):9090-9096. doi: 10.1021/acs.jmedchem.7b00933. PMID: 29019676