From Structure to Function: Cbl-b Inhibition in Cancer Immunotherapy
The human immune system serves as our body’s defense against harmful pathogens and cancer by identifying and eliminating abnormal cells. To maintain immune homeostasis several mechanisms are employed, which involve a wide range of proteins, including the Casitas B lymphoma-b (Cbl-b) protein, present in various immune cells. Cbl-b helps modulate immune responses by downregulating the activity of multiple immune cells. This inhibitory effect is significant in cancer development and progression as it promotes an immunosuppressive tumor environment. Therefore, Cbl-b is a prime target for cancer immunotherapy and treating other immune-related disorders, including infections and autoimmune diseases.
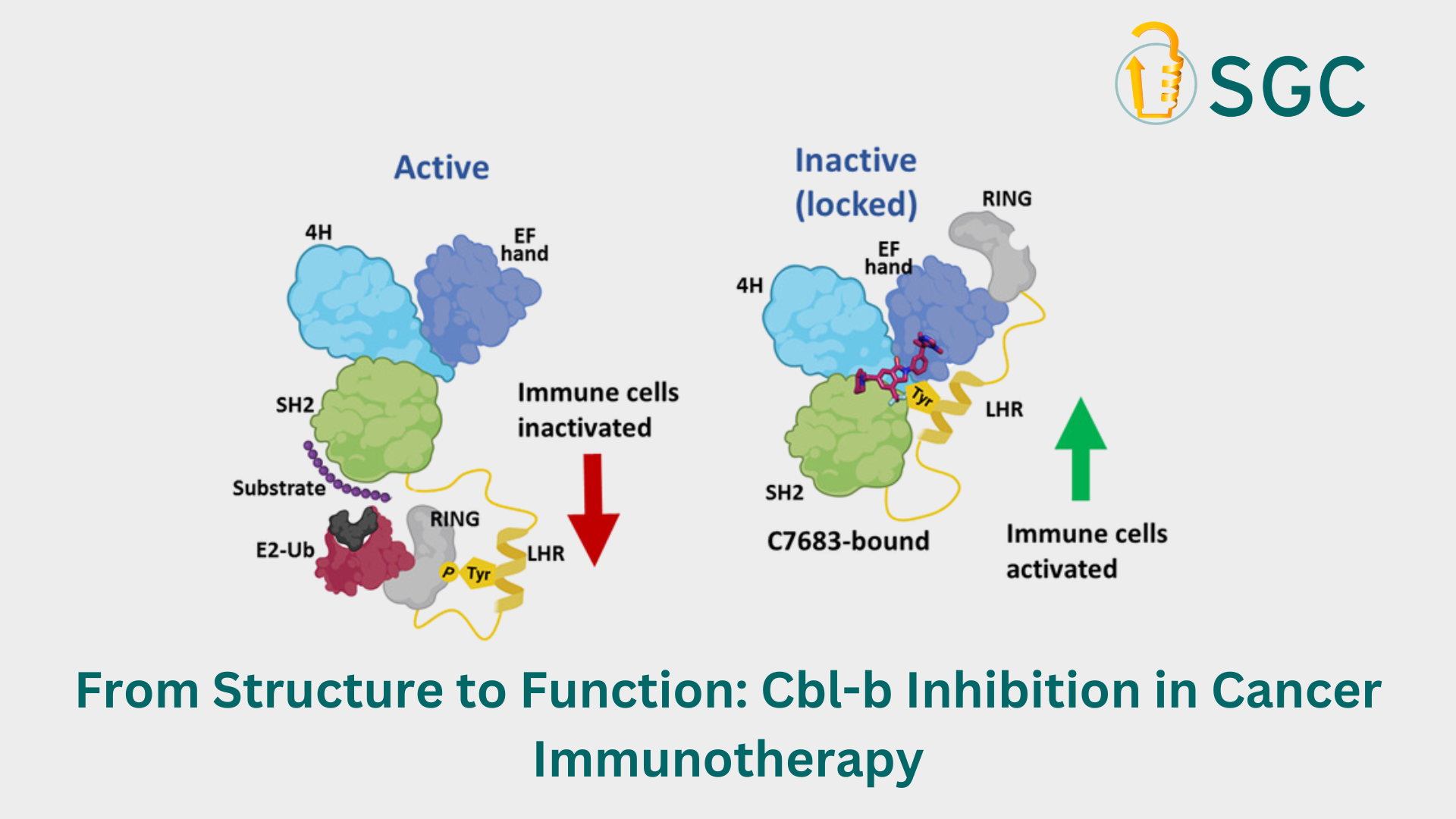
Inhibiting Cbl-b can activate the immune system, restoring its balance. While a few potent Cbl-b inhibitors have been reported, our understanding of how these inhibitors interact with Cbl-b is currently limited, and the mechanism of their action is not well understood. A recent example is the inhibitor Nx-1607, discovered by Nurix Therapeutics Inc., which is currently in clinical trials for treating solid tumor malignancies. NX-1607 targets the Cbl-b, preventing the ubiquitination and degradation of proteins vital for activating immune cells. This process stimulates immune cells in vitro and inhibits tumors in vivo, but the structural basis of this interaction is still unclear.
A study conducted by Levon Halabelian's group at SGC-Toronto used C7683, a compound from the Nx-1607 series, to investigate this interaction.
“Our study revealed that C7683 binds to Cbl-b at the interface of two subdomains (tyrosine kinase-binding domain (TKBD) and a short linker helix region (LHR)), thereby acting as an intramolecular glue and locking the protein in an inactive state. This inhibition prevents Cbl-b from activating and performing its normal regulatory functions, thus restoring the immune system's ability to combat tumors and infections”, shares Serah Kimani, lead author of this study and a research scientist in Halabelian's group.
The group developed structural data, methodologies, and biochemical and cellular assays, enhancing our understanding of Cbl-b function at a molecular level and potentially supporting future drug discovery efforts for selective Cbl-b inhibition. Additionally, their co-crystal structure of C7683-Cbl-b has allowed for in silico hit-finding efforts through the Critical Assessment of Computational Hit-finding Experiments (CACHE), a public-private partnership benchmarking initiative that supports the development of computational methods for hit-finding.
Read more: https://www.nature.com/articles/s42003-023-05655-8#Sec2
