This probe is available from Sigma and Cayman Chemical.
| Probe |
 |
PFI-4 |
A chemical probe for the bromodomains of the BRPF (BRomodomain and PHD Finger containing) family of proteins (BRPF1/2/3) has been discovered by the SGC. BRPF1, BRPF2 (BRD1) and BRPF3 are scaffolding proteins, assembling HAT complexes of the MOZ/MORF family (MOZ, Ybf2/Sas3, Sas2, and Tip60) (1). These MYST complexes have a tetrameric core containing BRPF, the tumour suppressor ING and Eaf6/EPC (enhancer of polycomb)-related scaffold subunits. MYST complexes play crucial roles in DNA repair, recombination, and replication as well as in transcription activation (2,3). MOZ is frequently translocated in AML (acute myeloid leukemia) and is required for HSC proliferation (4). Two BRPF1 isoforms (isoform A and B) can be generated by alternative splicing. In contrast to BRPF1B, the isoform A harbours a residue insertion in the ZA-loop that prevents binding to acetylated histone peptides (5).
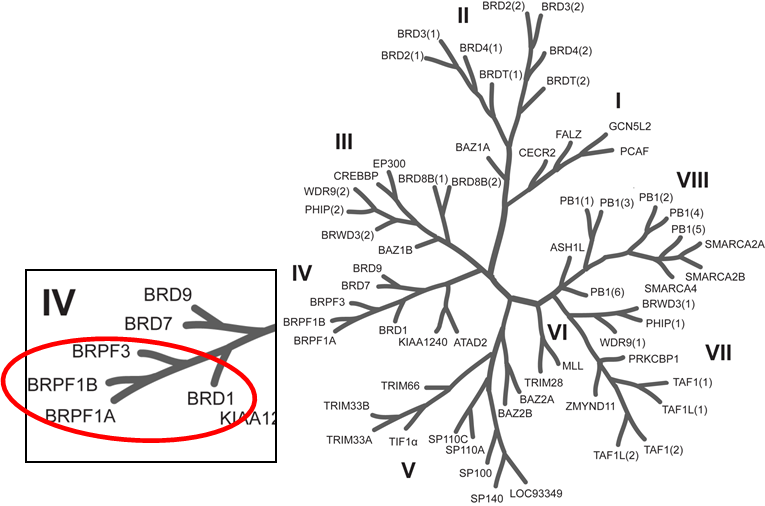
Phylogenetic tree of bromodomains and detailed view at the BRPF family.
A chemical probe for the bromodomain of the BRPF1B has been developed in collaboration with Pfizer. PFI-4 specifically binds to BRPF1B with a KD of 13 nM as determined by ITC. It reduces recovery time in U2OS cells transfected with a BRPF1B triple BRD construct with a nuclear localisation signal (NLS) in the FRAP assay at 500 nM, while showing no effect on BRPF1A. A NanoBRETTM cellular target engagement assay evaluating the interaction of BRPF1B with histones suggested IC50 of 240 nM for PFI-4.
| BRPF | Kd/nM (ITC) | IC50/nM (Alpha Screen) | TM Shift °C |
| BRPF1B | 13 | 172 | 9.4 |
| BRPF2 (BRD1) | 775 | 3600 | 2 |
PFI-4 specifically binds to BRPF1B with a KD of 13 nM as determined by ITC.
PFI-4 induced significant temperature shifts for BRPF1B (ΔTm of 9.4 °C). Weak interactions (2.0 °C) were also observed for BRD1 and CECR2, however the binding to BRD1 shows 60-fold selectivity with a KD of 775 nM and IC50 of 3600 nM. CECR2 was the only detected off-target outside family IV with only 2350 nM affinity, thus showing 167-fold selectivity.
Use PFI-4 at concentrations of around 1 µM. PFI-4 is non-toxic up to 50 μM in U2OS cells.
In a FRAP assay PFI-4 recovery time in U2OS cells transfected with a BRPF1B triple BRD construct with a nuclear localisation signal (NLS) at 500 nM. PFI-4 had no effect on the recovery time of BRPF1A.
In a NanoBRETTM cellular target engagement assay using isolated BRPF1B BRD with NLS and Halo-tagged histone H3.3 BRPF1B isoform showed a dose-dependent displacement from histone H3.3, with estimated cellular IC50 of 240 μM. No PFI-4 had no effect on the BRPF1A isoform.
PFI-4 induces re-localisation of BRPF1B resulting in a more uniform distribution in nuclei and expression in nucleoli as well as a greater expression in cytoplasm PFI-4 has no effect on the BRPF1A isoform.
| Probe |
 |
PFI-4 |
| Physical and chemical properties | |
|---|---|
| Molecular weight | 380.45 |
| Molecular formula | C21H24N4O3 |
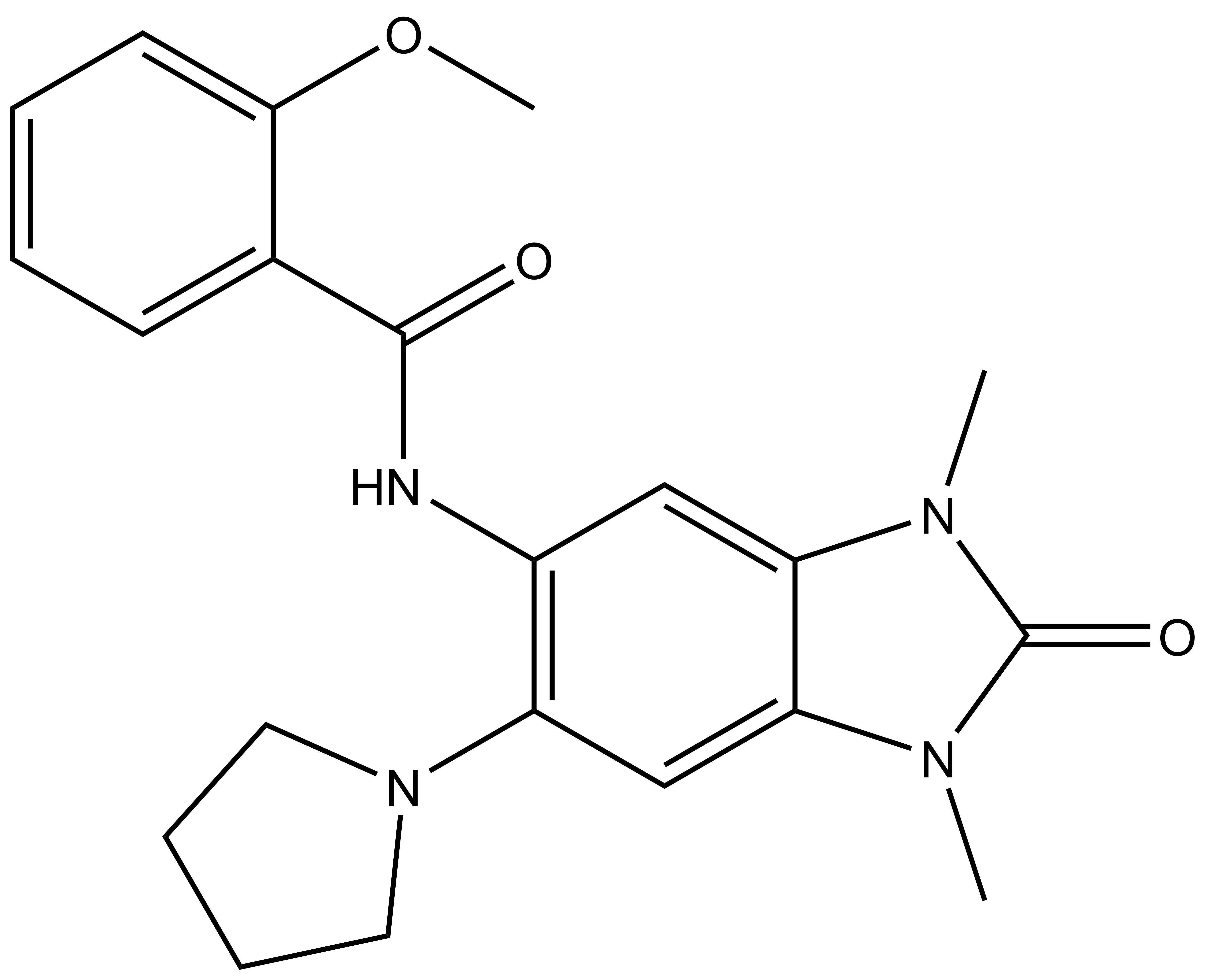
| IUPAC name | N-(1,3-dimethyl-2-oxo-6-(pyrrolidin-1-yl)-2,3-dihydro-1H-benzo[d]imidazol-5-yl)-2-methoxybenzamide |
| clogP | 2.23 |
| PSA | 65.12 |
| No. of chiral centres | 0 |
| No. of rotatable bonds | 5 |
| No. of hydrogen bond acceptors | 4 |
| No. of hydrogen bond donors | 1 |
| Storage | Stable as solid in the dark at -20°C. NB making aliquots rather than freeze-thawing is recommended |
| Dissolution | Soluble in DMSO |
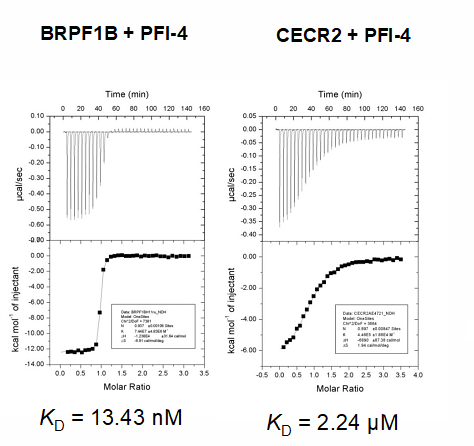
Dissociation constants of PFI-4 and BRDF1B or CECR2 interactions measured by isothermal titration calorimetry (ITC).
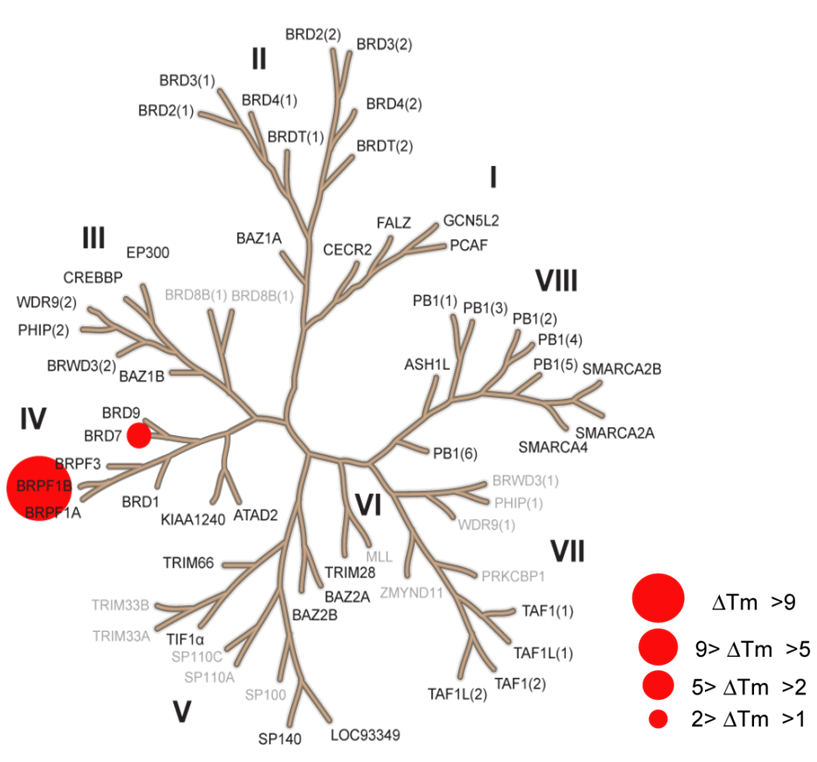
The temperature shifts mapped onto the phylogenetic tree using red circles corresponding to ΔTm as indicated in the figure.
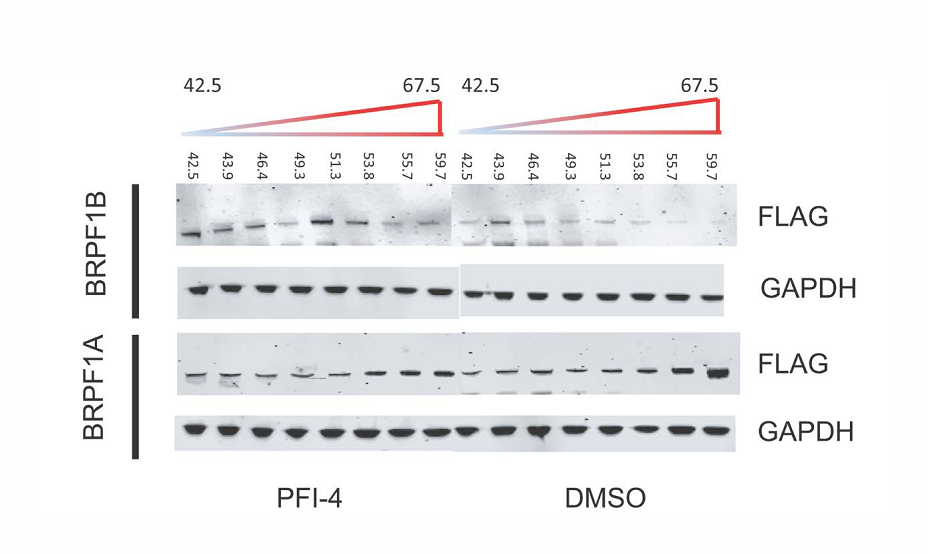
Thermal-stability of BRPF1A/B determined by CESTA at 1 µM PFI-4.
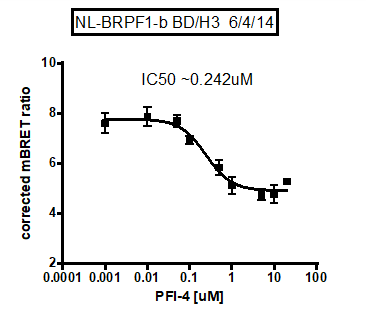
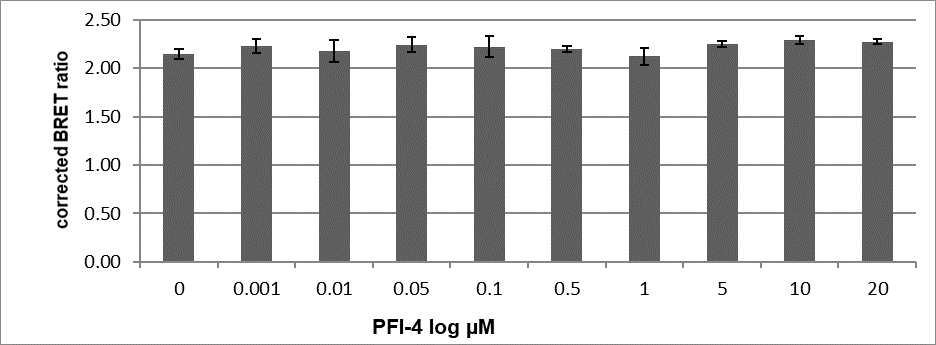
BRET assay using BRPF1A or BRPF1B-BRD with NLS (nuclear localisation signal) and H3.3 after treatment with PFI-4.
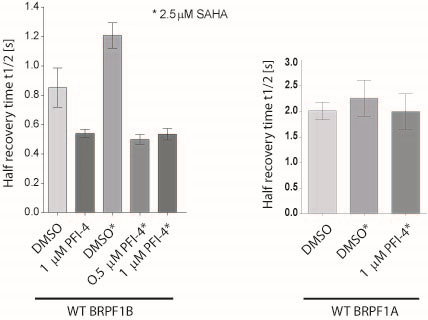
Half-times of fluorescence recovery (t1/2) after photo-bleaching measured for BRPF1B and BRPF1A treated either with or without SAHA and PFI-4 at indicated concentrations.
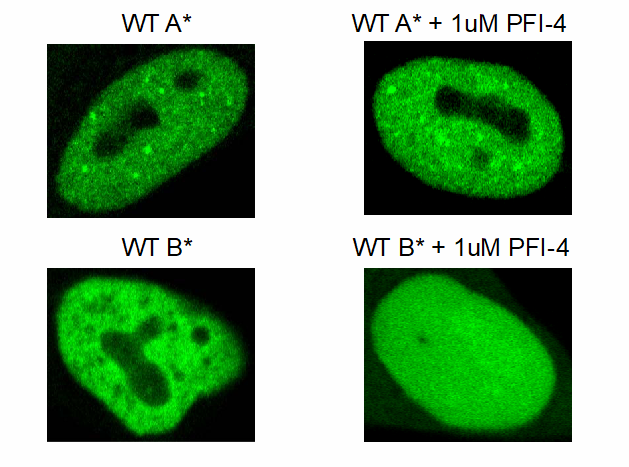
Confocal pictures of U2OS cells transfected with triple BRD–GFP construct of BRPF1B or BRPF1A containing NLS treated either with or without 1 μM PFI-4.
Work on this probe has been published in 'Selective targeting of Bromodomain-PHD fingers family (BRPF) bromodomains impairs osteoclast differentiation'.
Isothermal Titration Calorimetry (ITC)
All calorimetric experiments were performed on a VP-ITC micro-calorimeter (MicroCalTM, LLC Northampton, MA). Protein solutions were buffer exchanged by gel filtration or dialysis into buffer (20 mM Hepes pH 7.5, 150 mM NaCl, and 0.5 mM tris (2-carboxyethyl) phosphine (TCEP). All measurements were carried out at 288.15 K. All injections were performed using an initial injection of 2 µL followed by injections of 8 µL. The data were analysed with the MicroCal ORIGIN software package employing a single binding site model. The first data point was excluded from the analysis.
Temperature shift assay
Thermal melting experiments were carried out using a Stratagene Mx3005p Real Time PCR machine (Agilent Technologies). PFI-4 was added at a final concentration of 10 µM. SYPRO Orange (Molecular Probes) was added as a fluorescence probe at a dilution of 1:1000 as described (6).
AlphaScreen Assay
Assays were performed as described previously with minor modifications (7). All reagents were diluted in 25 mM HEPES, 100 mM NaCl, 0.1 % BSA, pH 7.4 supplemented with 0.05 % CHAPS and allowed to equilibrate to room temperature prior to addition to plates. An 11-point 1:2.0 serial dilutions of the ligands was prepared on lowvolume 384-well plates (ProxiPlateTM-384 Plus, PerkinElmer, USA), using LabCyte Eco liquid handler. Plates filled with 5 µL of the assay buffer followed by 7 µL of biotinylated peptide [H-YSGRGKacGGKacGLGKacGGAKacRHRK(Biotin)-OH for BRD1, BRD4, BRPF1B and BRPF3 or YQTARKSTGGK(ac)APRKQLATKAK(biotin)-OH for TIF1α] and Histagged protein to achieve final assay concentrations of 25-100 nM depending on the dose-response curve for each individual protein. Plates were sealed and incubated for a further 30 minutes, before the addition of 8 µM of the mixture of streptavidin-coated donor beads (12.5 µg/mL) and nickel chelate acceptor beads (12.5 µg/mL) under low light conditions. Plates were foil-sealed to protect from light, incubated at room temperature for 60 minutes and read on a PHERAstar FS plate reader (BMG Labtech, Germany) using an AlphaScreen 680 excitation/570 emission filter set. IC50 values were calculated in Prism 5 (GraphPad Software, USA) after normalization against corresponding DMSO controls.
Fluorescence Recovery After Photobleaching (FRAP) Assay
FRAP studies were performed using U20S cells expressing GFP-bound BRPF1a or BRPF1B (triple bromodomain). Six hours after transfection 2.5 µM SAHA (to increase global histone acetylation) was added and cells were treated with 1 µM or 5 µM of PFI-4 1 hour before imaging and half recovery times from the fluorescence signal of the bleached U2OS nuclei were plotted.
NanoBRET
U2OS cells were co-transfected with Histone H3.3-HaloTag and NanoLuc-BRPF1. Twenty hours post-transfection cells were collected, washed with PBS, and exchanged into media containing phenol red-free DMEM and 4% FBS in the absence (control sample) or the presence (experimental sample) of 100 nM NanoBRET 618 fluorescent ligand (Promega). Cells were then treated with an increasing dose of OF-1. Five minutes prior to reading, NanoBRET furimazine substrate (Promega) was added to both control and experimental samples and plates were read on a CLARIOstar (BMG) equipped with 450/80 nm bandpass and 610 nm longpass filters with a 0.5 sec reading setting. A corrected BRET ratio was calculated and is defined as the ratio of the emission at 610 nm/450 nm for experimental samples (i.e. those treated with NanoBRET fluorescent ligand) subtracted by and the emission at 610 nm/450 nm for control samples (not treated with NanoBRET fluorescent ligand). BRET ratios are expressed as milliBRET units (mBU), where 1 mBU corresponds to the corrected BRET ratio multiplied by 1000. Relative IC50 values were estimated by non-linear regression analysis of (log) concentration of each inhibitor versus milliBRET ratios (GraphPad Prism).