This probe hydrochloride) is available from Sigma (including its negative control), Tocris (including its negative control) and Cayman Chemical (including its negative control).
| Probe | Negative control | |
 |
|  |
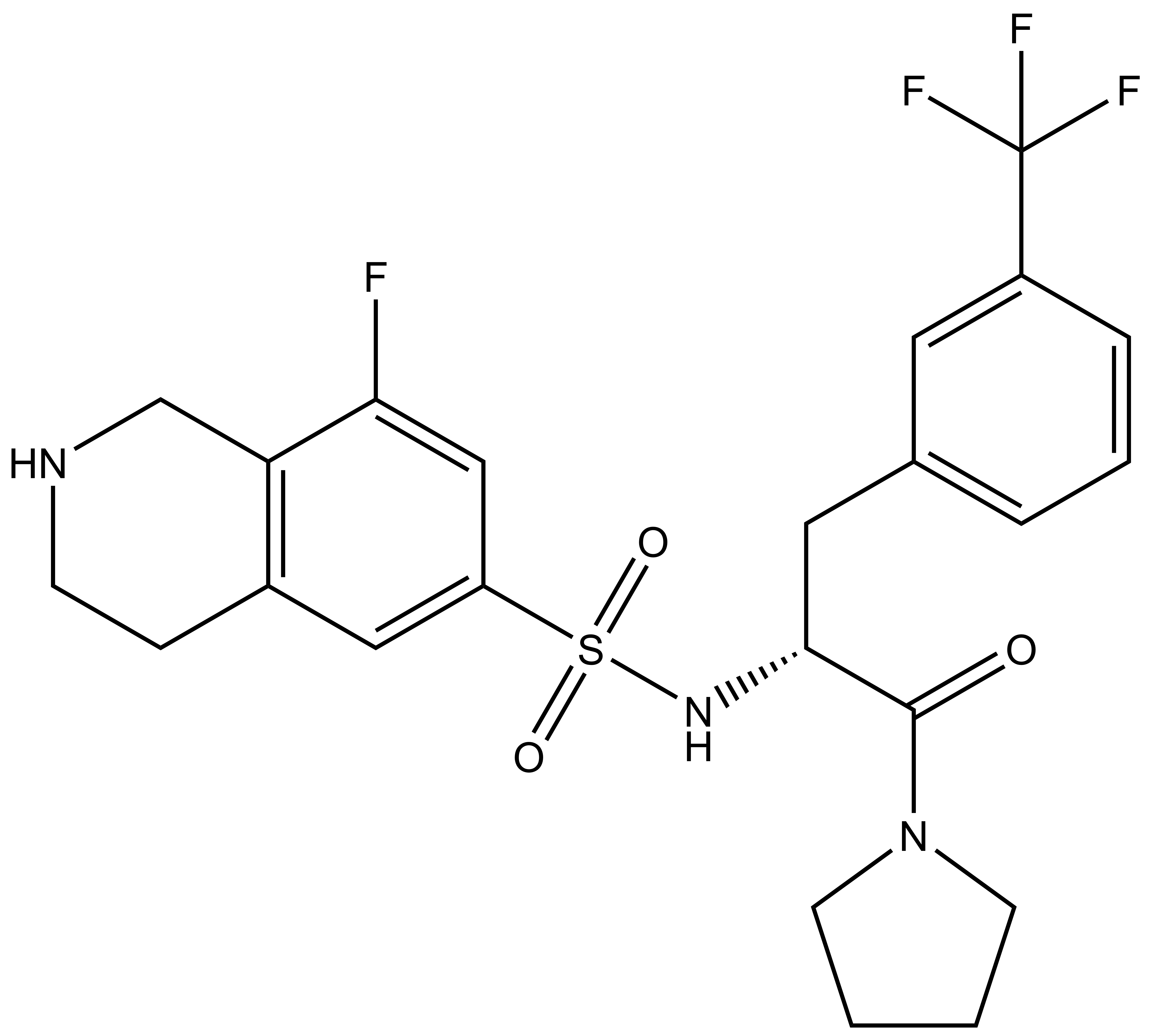
(R)-PFI-2 |
| (S)-PFI-2 |
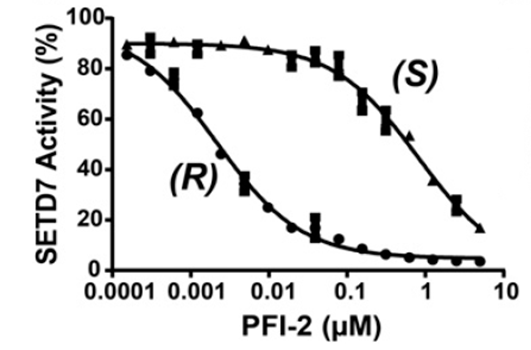
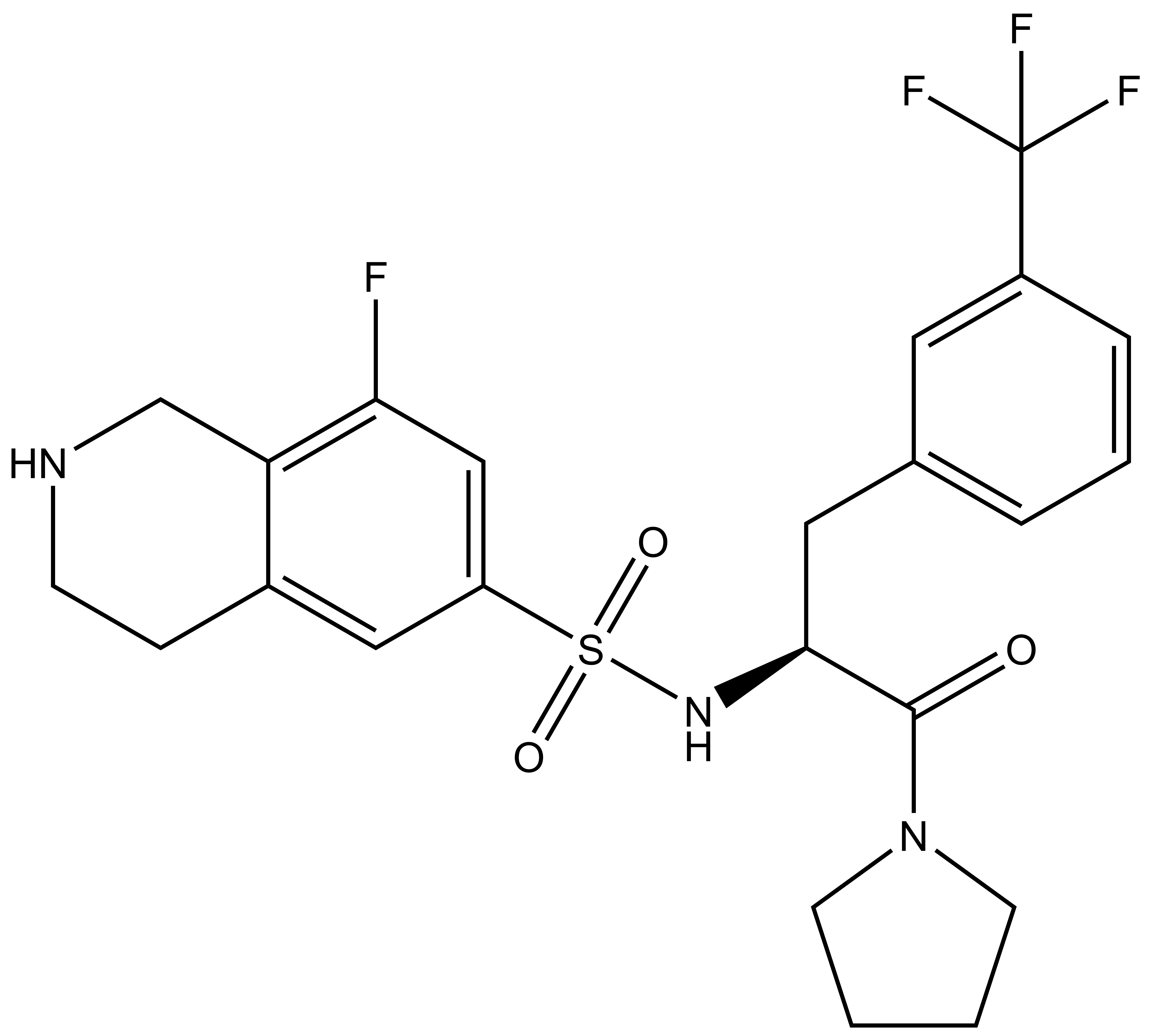
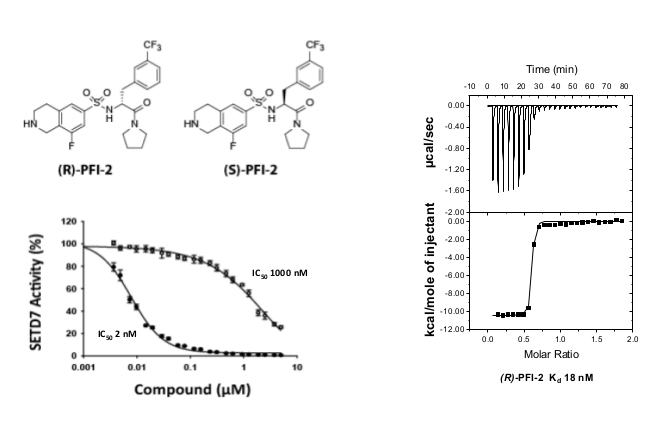
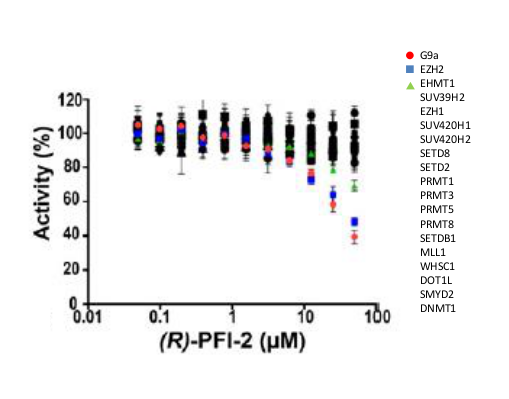
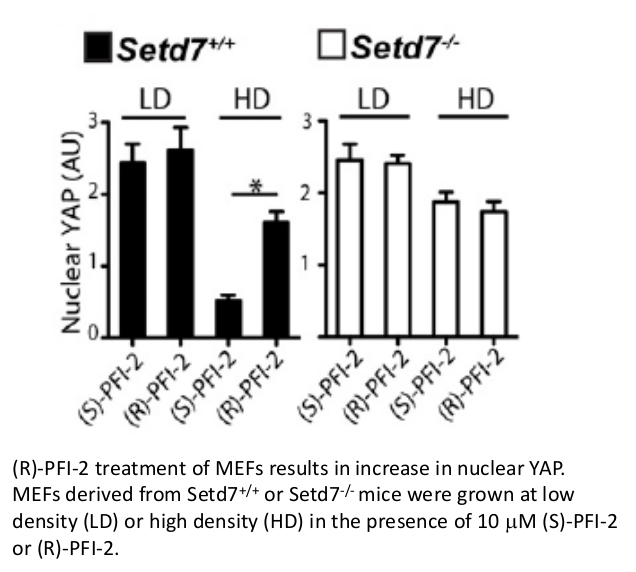
A collaboration between the SGC and Pfizer has resulted in the discovery of (R)-PFI-2, a chemical Probe for SETD7. PFI-2 is a potent inhibitor of SETD7 with IC50 2 nM and 1000-fold selectivity over other methyltransferases and other non-epigenetic targets. PFI-2 has been shown to bind to SETD7 by ITC (Kd=18nM) and biotinylated PFI-2 interacts with SETD7 in pull-down studies. Its enantiomer (S)-PFI-2 is 500-fold less active making it an excellent negative control. Treatment of low-density MEFs with (R)-PFI-2 resulted in higher nuclear YAP levels indicating an effect on the Hippo pathway.
| Probe | Negative control | |
 |
|  |
(R)-PFI-2 |
| (S)-PFI-2 |
| Physical and chemical properties for (S)-PFI-2 | |
| Molecular weight | 499.2 |
| Molecular formula | C23H25F4N3O3S |
| MollogP | 3.504 |
| PSA | 70.67 |
| No. of chiral centres | 1 |
| No. of rotatable bonds | 8 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 2 |
| Physical and chemical properties for (R)-PFI-2 | |
| Molecular weight | 499.2 |
| Molecular formula | C23H25F4N3O3S |
| MollogP | 3.504 |
| PSA | 70.67 |
| No. of chiral centres | 1 |
| No. of rotatable bonds | 8 |
| No. of hydrogen bond acceptors | 8 |
| No. of hydrogen bond donors | 2 |
Barsyte-Lovejoy D, Li F, Oudhoff MJ, Tatlock JH, Dong A, Zeng H, Wu H, Freeman SA, Schapira M, Senisterra GA, Kuznetsova E, Marcellus R, Allali-Hassani A, Kennedy S, Lambert JP, Couzens AL, Aman A, Gingras AC, Al-Awar R, Fish PV, Gerstenberger BS, Roberts L, Benn CL, Grimley RL, Braam MJ, Rossi FM, Sudol M, Brown PJ, Bunnage ME, Owen DR, Zaph C, Vedadi M, Arrowsmith CH. (R)-PFI-2 is a potent and selective inhibitor of SETD7 methyltransferase activity in cells. Proc Natl Acad Sci U S A. 2014 Sep 2;111(35):12853-8. doi: 10.1073/pnas.1407358111. Epub 2014 Aug 18.