
Dr. Dalia Barsyte-Lovejoy, PhD is an Assistant Professor at the Department of Pharmacology and Toxicology, UofT, and Principal Investigator at the SGC-Toronto, working to understand fundamental regulatory mechanisms of epigenetic proteins and their pharmacological modulation in cancer. The group’s research focuses on disease mechanisms, therapeutic targets, and chemical probe discovery, resulting in over 30 extensively characterized compounds that have helped shape the emerging field of epigenetics and enabled over 50 collaborative projects that are uncovering new epigenetic mechanisms in cancer and its treatment.
We are interested in understanding the mechanism of epigenetic regulators and posttranslational modifications that control cancer cell growth, differentiation, and therapy response. Protein lysine and arginine methyltransferases regulate transcription, genome stability, splicing, RNA metabolism, and other cell processes dictated by which substrates these enzymes methylate. Lysine methyltransferases such as EZH2 and NSD2 primarily methylate histones to establish repressive and active chromatin. In contrast, arginine methyltransferases have a broad scope of substrates ranging from histones to signaling molecules, enzymes, and structural proteins. Epigenetic chromatin regulation, transcriptome, and cellular signaling are fine-tuned by ubiquitin modification. Our work seeks to understand how these posttranslational modifications are misregulated in cancer and identify new therapeutic targets.
Through multidisciplinary research that includes cell and chemical biology, protein structural biology, and many collaborative studies with colleagues across industry and academia, the SGC chemical probes project has generated several probes for methyltransferases, ubiquitin ligases, and deubiquitylases. We are currently using these chemical probes to explore the cellular pathways in poor prognosis acute myeloid leukemia, pancreatic, lung and breast cancer.
Chemical probes as tools for cancer target discovery
|
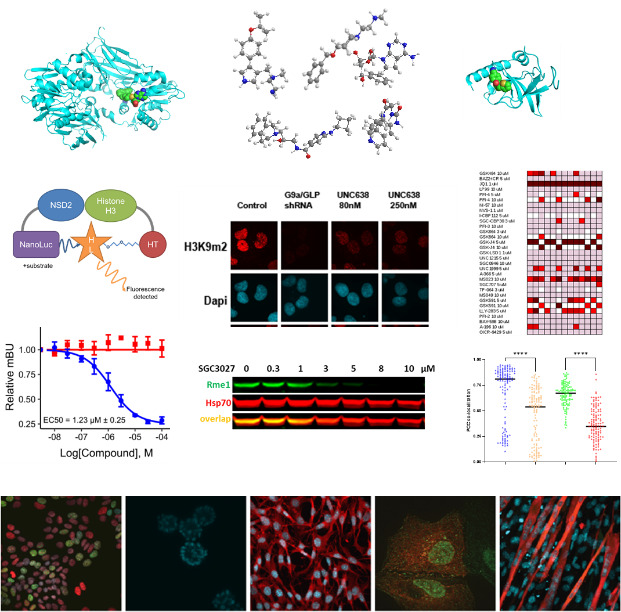
To study epigenetic modifier proteins, we need genetic and pharmacological tools. Chemical probe compounds that potently and selectively inhibit or degrade the target proteins in cells provide tools for modulating activating/repressing histone marks and other cellular signaling pathways. By discovering and using chemical probes, we expand our understanding of the protein function and its therapeutic utility to establish a biological rationale in cancer therapy.
|
Link to Open Lab notebooks that features science community posts on our various projects https://openlabnotebooks.org/
Wu Q, Nie DY, Ba-Alawi W, Ji Y, Zhang Z, Cruickshank J, Haight J, Ciamponi FE, Chen J, Duan S, Shen Y, Liu J, Marhon SA, Mehdipour P, Szewczyk MM, Dogan-Artun N, Chen W, Zhang LX, Deblois G, Prinos P, Massirer KB, Barsyte-Lovejoy D, Jin J, De Carvalho DD, Haibe-Kains B, Wang X, Cescon DW, Lupien M, Arrowsmith CH
Nat Chem Biol. 2022-5-16 . .doi: 10.1038/s41589-022-01024-4
PMID: 35578032Vu V, Szewczyk MM, Nie DY, Arrowsmith CH, Barsyte-Lovejoy D
Annu Rev Biochem. 2022-4-1 . .doi: 10.1146/annurev-biochem-032620-105344
PMID: 35363509Srour N, Villarreal OD, Hardikar S, Yu Z, Preston S, Miller WH, Szewczyk MM, Barsyte-Lovejoy D, Xu H, Chen T, Del Rincón SV, Richard S
Cell Rep. 2022-3-29 . 38(13):110582 .doi: 10.1016/j.celrep.2022.110582
PMID: 35354055Szewczyk MM, Luciani GM, Vu V, Murison A, Dilworth D, Barghout SH, Lupien M, Arrowsmith CH, Minden MD, Barsyte-Lovejoy D
Redox Biol. 2022-3-11 . 51:102282 .doi: 10.1016/j.redox.2022.102282
PMID: 35305370Dilworth D, Hanley RP, Ferreira de Freitas R, Allali-Hassani A, Zhou M, Mehta N, Marunde MR, Ackloo S, Carvalho Machado RA, Khalili Yazdi A, Owens DDG, Vu V, Nie DY, Alqazzaz M, Marcon E, Li F, Chau I, Bolotokova A, Qin S, Lei M, Liu Y, Szewczyk MM, Dong A, Kazemzadeh S, Abramyan T, Popova IK, Hall NW, Meiners MJ, Cheek MA, Gibson E, Kireev D, Greenblatt JF, Keogh MC, Min J, Brown PJ, Vedadi M, Arrowsmith CH, Barsyte-Lovejoy D, James LI, Schapira M
Nat Chem Biol. 2021-11-15 . .doi: 10.1038/s41589-021-00898-0
PMID: 34782742Szewczyk MM, Vu V, Barsyte-Lovejoy D
J Vis Exp. 2021-8-7 . .doi: 10.3791/62418
PMID: 34424246Halabelian L, Barsyte-Lovejoy D
Life (Basel). 2021-7-30 . 11(8): .doi: 10.3390/life11080768
PMID: 34440512Gagnon A, Mélin L, Abdullayev S, Fnaiche A, Vu V, Gonzalez Suarez N, Zeng H, Szewczyk M, Li F, Sinisterra G, Allali-Hassani A, Chau I, Dong A, Woo S, Annabi B, Halabelian L, LaPlante S, Vedadi M, Barsyte-Lovejoy D, Santhakumar V
ChemMedChem. 2021-6-23 . .doi: 10.1002/cmdc.202100432
PMID: 34164919Gradl S, Steuber H, Weiske J, Szewczyk MM, Schmees N, Siegel S, Stoeckigt D, Christ CD, Li F, Organ S, Abbey M, Kennedy S, Chau I, Trush V, Barsyte-Lovejoy D, Brown PJ, Vedadi M, Arrowsmith C, Husemann M, Badock V, Bauser M, Haegebarth A, Hartung IV, Stresemann C
SLAS Discov. 2021-6-21 . 24725552211019409 .doi: 10.1177/24725552211019409
PMID: 34154424Dölle A, Adhikari B, Krämer A, Weckesser J, Berner N, Berger LM, Diebold M, Szewczyk MM, Barsyte-Lovejoy D, Arrowsmith CH, Gebel J, Löhr F, Dötsch V, Eilers M, Heinzlmeir S, Kuster B, Sotriffer C, Wolf E, Knapp S
J Med Chem. 2021-5-13 . .doi: 10.1021/acs.jmedchem.1c00146
PMID: 33980013