
Dr. Dalia Barsyte-Lovejoy, PhD is an Assistant Professor at the Department of Pharmacology and Toxicology, UofT, and Principal Investigator at the SGC-Toronto, working to understand fundamental regulatory mechanisms of epigenetic proteins and their pharmacological modulation in cancer. The group’s research focuses on disease mechanisms, therapeutic targets, and chemical probe discovery, resulting in over 30 extensively characterized compounds that have helped shape the emerging field of epigenetics and enabled over 50 collaborative projects that are uncovering new epigenetic mechanisms in cancer and its treatment.
We are interested in understanding the mechanism of epigenetic regulators and posttranslational modifications that control cancer cell growth, differentiation, and therapy response. Protein lysine and arginine methyltransferases regulate transcription, genome stability, splicing, RNA metabolism, and other cell processes dictated by which substrates these enzymes methylate. Lysine methyltransferases such as EZH2 and NSD2 primarily methylate histones to establish repressive and active chromatin. In contrast, arginine methyltransferases have a broad scope of substrates ranging from histones to signaling molecules, enzymes, and structural proteins. Epigenetic chromatin regulation, transcriptome, and cellular signaling are fine-tuned by ubiquitin modification. Our work seeks to understand how these posttranslational modifications are misregulated in cancer and identify new therapeutic targets.
Through multidisciplinary research that includes cell and chemical biology, protein structural biology, and many collaborative studies with colleagues across industry and academia, the SGC chemical probes project has generated several probes for methyltransferases, ubiquitin ligases, and deubiquitylases. We are currently using these chemical probes to explore the cellular pathways in poor prognosis acute myeloid leukemia, pancreatic, lung and breast cancer.
Chemical probes as tools for cancer target discovery
|
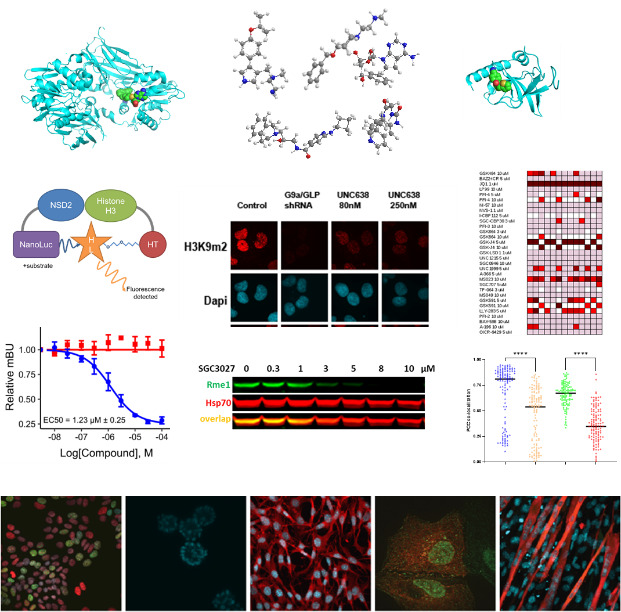
To study epigenetic modifier proteins, we need genetic and pharmacological tools. Chemical probe compounds that potently and selectively inhibit or degrade the target proteins in cells provide tools for modulating activating/repressing histone marks and other cellular signaling pathways. By discovering and using chemical probes, we expand our understanding of the protein function and its therapeutic utility to establish a biological rationale in cancer therapy.
|
Link to Open Lab notebooks that features science community posts on our various projects https://openlabnotebooks.org/
Taylor AP, Szewczyk MM, Kennedy S, Trush VV, Wu H, Zeng H, Dong A, de Freitas RF, Tatlock JH, Kumpf RA, Wythes M, Casimiro-Garcia A, Denny RA, Parikh MD, Li F, Barsyte-Lovejoy D, Schapira M, Vedadi M, Brown P, Arrowsmith CH, Owen DR
J. Med. Chem.. 2019-8-15 . .doi: 10.1021/acs.jmedchem.9b00112
PMID: 31415173Fong JY, Pignata L, Goy PA, Kawabata KC, Lee SC, Koh CM, Musiani D, Massignani E, Kotini AG, Penson A, Wun CM, Shen Y, Schwarz M, Low DH, Rialdi A, Ki M, Wollmann H, Mzoughi S, Gay F, Thompson C, Hart T, Barbash O, Luciani GM, Szewczyk MM, Wouters BJ, Delwel R, Papapetrou EP, Barsyte-Lovejoy D, Arrowsmith CH, Minden MD, Jin J, Melnick A, Bonaldi T, Abdel-Wahab O, Guccione E
Cancer Cell. 2019-8-12 . 36(2):194-209.e9 .doi: 10.1016/j.ccell.2019.07.003
PMID: 31408619Sin-Chan P, Mumal I, Suwal T, Ho B, Fan X, Singh I, Du Y, Lu M, Patel N, Torchia J, Popovski D, Fouladi M, Guilhamon P, Hansford JR, Leary S, Hoffman LM, Mulcahy Levy JM, Lassaletta A, Solano-Paez P, Rivas E, Reddy A, Gillespie GY, Gupta N, Van Meter TE, Nakamura H, Wong TT, Ra YS, Kim SK, Massimi L, Grundy RG, Fangusaro J, Johnston D, Chan J, Lafay-Cousin L, Hwang EI, Wang Y, Catchpoole D, Michaud J, Ellezam B, Ramanujachar R, Lindsay H, Taylor MD, Hawkins CE, Bouffet E, Jabado N, Singh SK, Kleinman CL, Barsyte-Lovejoy D, Li XN, Dirks PB, Lin CY, Mack SC, Rich JN, Huang A
Cancer Cell. 2019-7-8 . 36(1):51-67.e7 .doi: 10.1016/j.ccell.2019.06.002
PMID: 31287992Böttcher J, Dilworth D, Reiser U, Neumüller RA, Schleicher M, Petronczki M, Zeeb M, Mischerikow N, Allali-Hassani A, Szewczyk MM, Li F, Kennedy S, Vedadi M, Barsyte-Lovejoy D, Brown PJ, Huber KVM, Rogers CM, Wells CI, Fedorov O, Rumpel K, Zoephel A, Mayer M, Wunberg T, Böse D, Zahn S, Arnhof H, Berger H, Reiser C, Hörmann A, Krammer T, Corcokovic M, Sharps B, Winkler S, Häring D, Cockcroft XL, Fuchs JE, Müllauer B, Weiss-Puxbaum A, Gerstberger T, Boehmelt G, Vakoc CR, Arrowsmith CH, Pearson M, McConnell DB
Nat. Chem. Biol.. 2019-7-8 . .doi: 10.1038/s41589-019-0310-x
PMID: 31285596Xiong Y, Greschik H, Johansson C, Seifert L, Bacher J, Park KS, Babault N, Martini ML, Fagan V, Li F, Chau I, Christott T, Dilworth D, Barsyte-Lovejoy D, Vedadi M, Arrowsmith CH, Brennan PE, Fedorov O, Jung M, Farnie G, Liu J, Oppermann UCT, Schüle R, Jin J
J. Med. Chem.. 2019-7-1 . .doi: 10.1021/acs.jmedchem.9b00522
PMID: 31260300Dilworth D, Barsyte-Lovejoy D
Cell. Mol. Life Sci.. 2019-5-18 . .doi: 10.1007/s00018-019-03147-9
PMID: 31104094Lima-Fernandes E, Murison A, da Silva Medina T, Wang Y, Ma A, Leung C, Luciani GM, Haynes J, Pollett A, Zeller C, Duan S, Kreso A, Barsyte-Lovejoy D, Wouters BG, Jin J, Carvalho DD, Lupien M, Arrowsmith CH, O'Brien CA
Nat Commun. 2019-3-29 . 10(1):1436 .doi: 10.1038/s41467-019-09309-4
PMID: 30926792Husić M, Barsyte-Lovejoy D, Lovejoy DA
Front Endocrinol (Lausanne). 2019-2-19 . 10:22 .doi: 10.3389/fendo.2019.00022
PMID: 30774623Scheer S, Ackloo S, Medina TS, Schapira M, Li F, Ward JA, Lewis AM, Northrop JP, Richardson PL, Kaniskan HÜ, Shen Y, Liu J, Smil D, McLeod D, Zepeda-Velazquez CA, Luo M, Jin J, Barsyte-Lovejoy D, Huber KVM, De Carvalho DD, Vedadi M, Zaph C, Brown PJ, Arrowsmith CH
Nat Commun. 2019-1-3 . 10(1):19 .doi: 10.1038/s41467-018-07905-4
PMID: 30604761Ivanochko D, Halabelian L, Henderson E, Savitsky P, Jain H, Marcon E, Duan S, Hutchinson A, Seitova A, Barsyte-Lovejoy D, Filippakopoulos P, Greenblatt J, Lima-Fernandes E, Arrowsmith CH
Nucleic Acids Res.. 2018-11-20 . .doi: 10.1093/nar/gky1192
PMID: 30462309